Physical Chemistry
Some Basic Concepts of Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveStructure of Atom
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseGaseous State
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Kinetics and Nuclear Chemistry
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveInorganic Chemistry
Periodic Table & Periodicity
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Bonding & Molecular Structure
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseOrganic Chemistry
Basics of Organic Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveAldehydes, Ketones and Carboxylic Acids
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)Chemistry in Everyday Life
MCQ (Single Correct Answer)1
JEE Advanced 2025 Paper 1 Online
Numerical
+4
-0
In an electrochemical cell, dichromate ions in aqueous acidic medium are reduced to Cr3+. The current (in amperes) that flows through the cell for 48.25 minutes to produce 1 mole of Cr3+ is ______.
Use: 1 Faraday = 96500 C mol−1
Your input ____
2
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1
Consider the strong electrolytes $Z_{m} X_{n}, U_{m} Y_{p}$ and $V_{m} X_{n}$. Limiting molar conductivity ( $\Lambda^{0}$ ) of $\mathrm{U}_{\mathrm{m}} \mathrm{Y}_{\mathrm{p}}$ and $\mathrm{V}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}}$ are 250 and $440 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, respectively. The value of $(\mathrm{m}+\mathrm{n}+\mathrm{p})$ is
Given:
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.
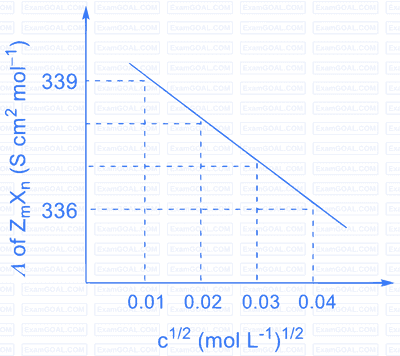
Given:
| Ion | $\mathrm{Z}^{\mathrm{n}+}$ | $\mathrm{U}^{\mathrm{p}+}$ | $\mathrm{V}^{\mathrm{n}+}$ | $\mathrm{X}^{\mathrm{m}-}$ | $\mathrm{Y}^{\mathrm{m}-}$ |
|---|---|---|---|---|---|
| $\lambda^{0}\left(\mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}\right)$ | $50.0$ | $25.0$ | $100.0$ | $80.0$ | $100.0$ |
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.

Your input ____
3
JEE Advanced 2022 Paper 1 Online
Numerical
+3
-0
The reduction potential $\left(E^{0}\right.$, in $\left.\mathrm{V}\right)$ of $\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})$ is __________.
[Given: $E_{\left(\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{MnO}_{2}(\mathrm{~s})\right)}^{0}=1.68 \mathrm{~V} ; E_{\left(\mathrm{MnO}_{2}(\mathrm{~s}) / \mathrm{Mn}^{2+}(\mathrm{aq})\right)}^{0}=1.21 \mathrm{~V} ; E_{\left(\mathrm{Mn}^{2+}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})\right)}^{0}=-1.03 \mathrm{~V}$ ]
Your input ____
4
JEE Advanced 2021 Paper 2 Online
Numerical
+2
-0
At 298 K, the limiting molar conductivity of a weak monobasic acid is 4 $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, for an aqueous solution of the acid the degree of dissociation is $$\alpha$$ and the molar conductivity is y $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, upon 20 times dilution with water, the molar conductivity of the solution becomes 3y $$\times$$ 102 S cm2 mol$$-$$1.
The value of $$\alpha$$ is __________.
The value of $$\alpha$$ is __________.
Your input ____
Questions Asked from Numerical
JEE Advanced 2025 Paper 2 Online (1) JEE Advanced 2025 Paper 1 Online (1) JEE Advanced 2022 Paper 2 Online (1) JEE Advanced 2022 Paper 1 Online (1) JEE Advanced 2021 Paper 2 Online (2) JEE Advanced 2020 Paper 1 Offline (1) JEE Advanced 2018 Paper 2 Offline (1) JEE Advanced 2018 Paper 1 Offline (1) JEE Advanced 2017 Paper 1 Offline (1) JEE Advanced 2015 Paper 2 Offline (1) JEE Advanced 2015 Paper 1 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Electricity
Modern Physics
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Coordinate Geometry