Physical Chemistry
Some Basic Concepts of Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveStructure of Atom
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseGaseous State
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Kinetics and Nuclear Chemistry
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveInorganic Chemistry
Periodic Table & Periodicity
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Bonding & Molecular Structure
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseOrganic Chemistry
Basics of Organic Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveAldehydes, Ketones and Carboxylic Acids
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)Chemistry in Everyday Life
MCQ (Single Correct Answer)1
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0
One mole of an ideal gas at 900 K, undergoes two reversible processes, I followed by II, as shown below. If the work done by the gas in the two processes are same, the value of $$\ln {{{V_3}} \over {{V_2}}}$$ is _________.
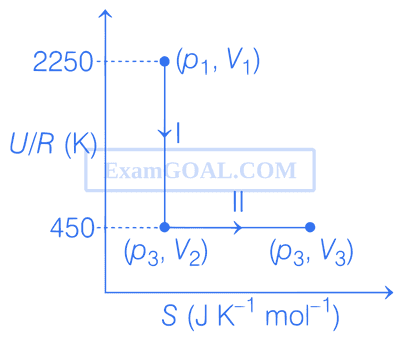
(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)

(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)
Your input ____
2
JEE Advanced 2021 Paper 1 Online
Numerical
+2
-0
For the reaction, X(s) $$\rightleftharpoons$$ Y(s) + Z(g), the plot of $$\ln {{pz} \over {{p^\theta }}}$$ versus $${{{{10}^4}} \over T}$$ is given below (in solid line), where pz is the pressure (in bar) of the gas Z at temperature T and $${{p^\theta }}$$ = 1 bar.
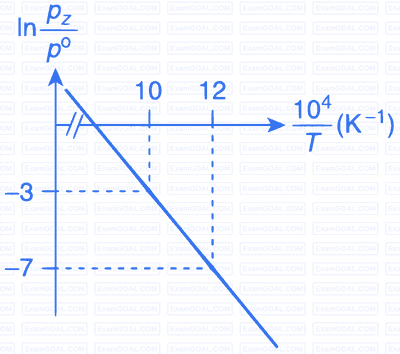
(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)

(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)
The value of standard enthalpy, $$\Delta$$Ho (in kJ mol$$-$$1) for the given reaction is _______.
Your input ____
3
JEE Advanced 2021 Paper 1 Online
Numerical
+2
-0
For the reaction, X(s) $$\rightleftharpoons$$ Y(s) + Z(g), the plot of $$\ln {{pz} \over {{p^\theta }}}$$ versus $${{{{10}^4}} \over T}$$ is given below (in solid line), where pz is the pressure (in bar) of the gas Z at temperature T and $${{p^\theta }}$$ = 1 bar.

(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)

(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)
The value of $$\Delta$$S$$\theta$$ (in J K$$-$$1 mol$$-$$1) for the given reaction, at 1000 K is _________.
Your input ____
4
JEE Advanced 2020 Paper 2 Offline
Numerical
+4
-0
Tin is obtained from cassiterite by reduction with coke. Use the data given below to determine the minimum temperature (in K) at which the reduction of cassiterite by coke would take place.
At $$298K:{\Delta _f}H^\circ [Sn{O_2}(s)] = - 581.0$$ mol-1,
$$\eqalign{ & {\Delta _f}H^\circ [(C{O_2})(g)] = - 394.0\,kJ\,mol{ ^{-1}} \cr & S^\circ [Sn{O_2}(s)] = 56.0J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [Sn(s)] = 52.0\,J\,K{ ^{-1}}mo{l^{ - 1}} \cr & S^\circ [C(s)] = 6.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [C{O_2}(g)] = 210.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr} $$
Assume that, the enthalpies and the entropies are temperature independent.
At $$298K:{\Delta _f}H^\circ [Sn{O_2}(s)] = - 581.0$$ mol-1,
$$\eqalign{ & {\Delta _f}H^\circ [(C{O_2})(g)] = - 394.0\,kJ\,mol{ ^{-1}} \cr & S^\circ [Sn{O_2}(s)] = 56.0J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [Sn(s)] = 52.0\,J\,K{ ^{-1}}mo{l^{ - 1}} \cr & S^\circ [C(s)] = 6.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [C{O_2}(g)] = 210.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr} $$
Assume that, the enthalpies and the entropies are temperature independent.
Your input ____
Questions Asked from Numerical
JEE Advanced 2025 Paper 1 Online (1) JEE Advanced 2024 Paper 1 Online (1) JEE Advanced 2023 Paper 2 Online (2) JEE Advanced 2023 Paper 1 Online (2) JEE Advanced 2022 Paper 1 Online (1) JEE Advanced 2021 Paper 2 Online (1) JEE Advanced 2021 Paper 1 Online (2) JEE Advanced 2020 Paper 2 Offline (1) JEE Advanced 2018 Paper 2 Offline (1) IIT-JEE 2010 Paper 2 Offline (1) IIT-JEE 2009 Paper 2 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Electricity
Modern Physics
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Coordinate Geometry