Physical Chemistry
Some Basic Concepts of Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveStructure of Atom
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseGaseous State
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Kinetics and Nuclear Chemistry
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveInorganic Chemistry
Periodic Table & Periodicity
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Bonding & Molecular Structure
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseOrganic Chemistry
Basics of Organic Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveAldehydes, Ketones and Carboxylic Acids
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)Chemistry in Everyday Life
MCQ (Single Correct Answer)1
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1
The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.
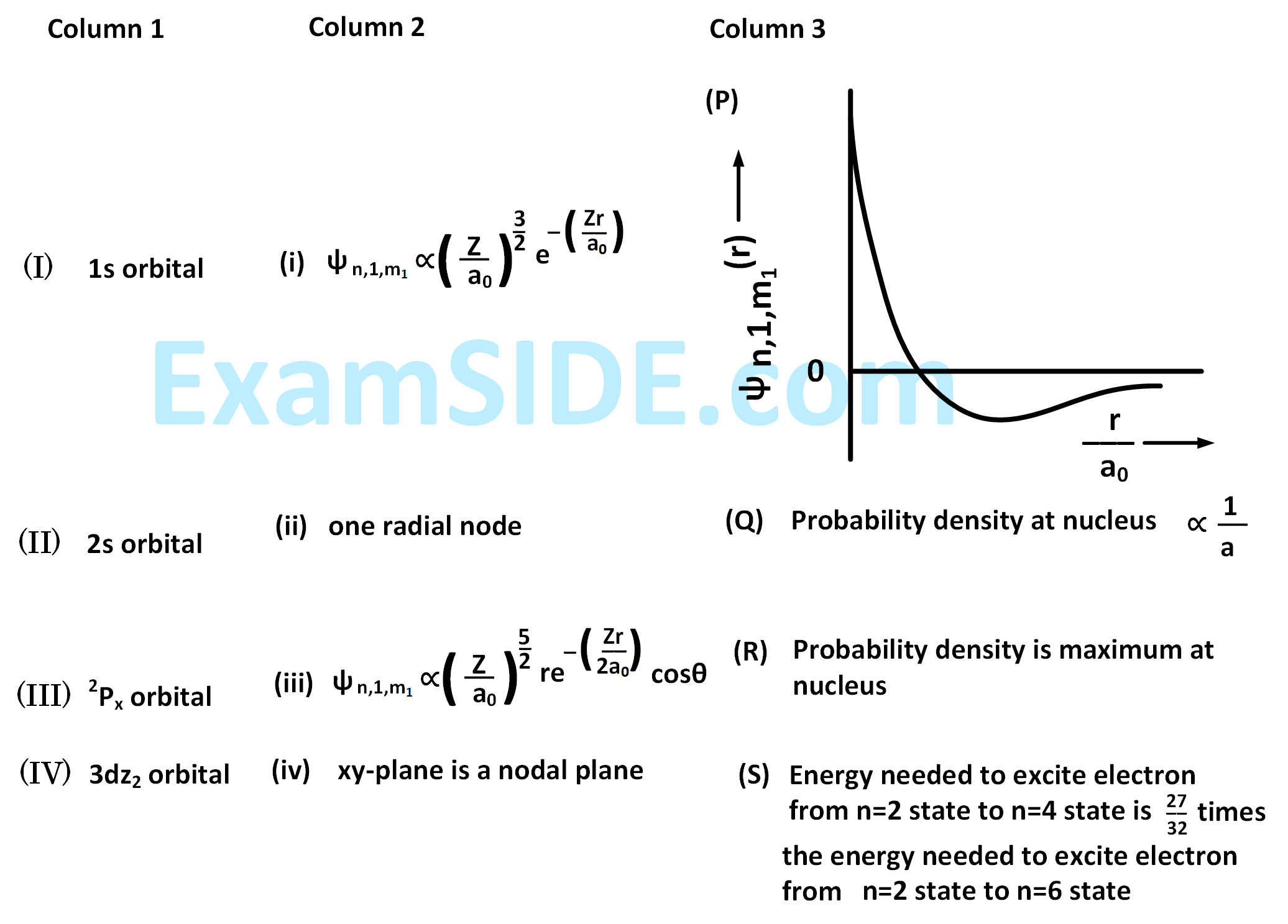

For the given orbital in Column 1, the only CORRECT combination for any hydrogen-like species is :
2
JEE Advanced 2016 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1
P is the probability of finding the 1s electron of hydrogen atom in a spherical shell of infinitesimal thickness, dr, at a distance r from the nucleus. The volume of this shell is $$4\pi r^2dr$$. The quantitative ketch of the dependence of P on r is
3
IIT-JEE 2012 Paper 1 Offline
MCQ (Single Correct Answer)
+4
-1
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is [$$\alpha_0$$ is Bohr radius]
4
IIT-JEE 2010 Paper 2 Offline
MCQ (Single Correct Answer)
+2
-0.5
The hydrogen like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
The state S1 is :
The state S1 is :
Questions Asked from MCQ (Single Correct Answer)
JEE Advanced 2024 Paper 2 Online (1) JEE Advanced 2019 Paper 2 Offline (2) JEE Advanced 2017 Paper 1 Offline (3) JEE Advanced 2016 Paper 1 Offline (1) IIT-JEE 2012 Paper 1 Offline (1) IIT-JEE 2010 Paper 2 Offline (3) IIT-JEE 2008 Paper 2 Offline (1) IIT-JEE 2008 Paper 1 Offline (1) IIT-JEE 2006 (1) IIT-JEE 2005 Screening (1) IIT-JEE 2004 Screening (1) IIT-JEE 2003 Screening (1) IIT-JEE 2002 Screening (2) IIT-JEE 2001 Screening (2) IIT-JEE 2000 Screening (2) IIT-JEE 1999 (1) IIT-JEE 1998 (2) IIT-JEE 1997 (1) IIT-JEE 1996 (1) IIT-JEE 1995 Screening (1) IIT-JEE 1992 (2) IIT-JEE 1989 (2) IIT-JEE 1988 (3) IIT-JEE 1986 (4) IIT-JEE 1985 (3) IIT-JEE 1984 (3) IIT-JEE 1983 (3) IIT-JEE 1982 (1) IIT-JEE 1981 (1) IIT-JEE 1979 (1)
JEE Advanced Subjects
Physics
Mechanics
Electricity
Modern Physics
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Coordinate Geometry



