Physical Chemistry
Some Basic Concepts of Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveStructure of Atom
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseGaseous State
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Kinetics and Nuclear Chemistry
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveInorganic Chemistry
Periodic Table & Periodicity
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseChemical Bonding & Molecular Structure
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveTrue of FalseOrganic Chemistry
Basics of Organic Chemistry
Fill in the BlanksNumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)SubjectiveAldehydes, Ketones and Carboxylic Acids
NumericalMCQ (Single Correct Answer)MCQ (Multiple Correct Answer)Chemistry in Everyday Life
MCQ (Single Correct Answer)1
JEE Advanced 2019 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.

Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
2
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-0.75
The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.
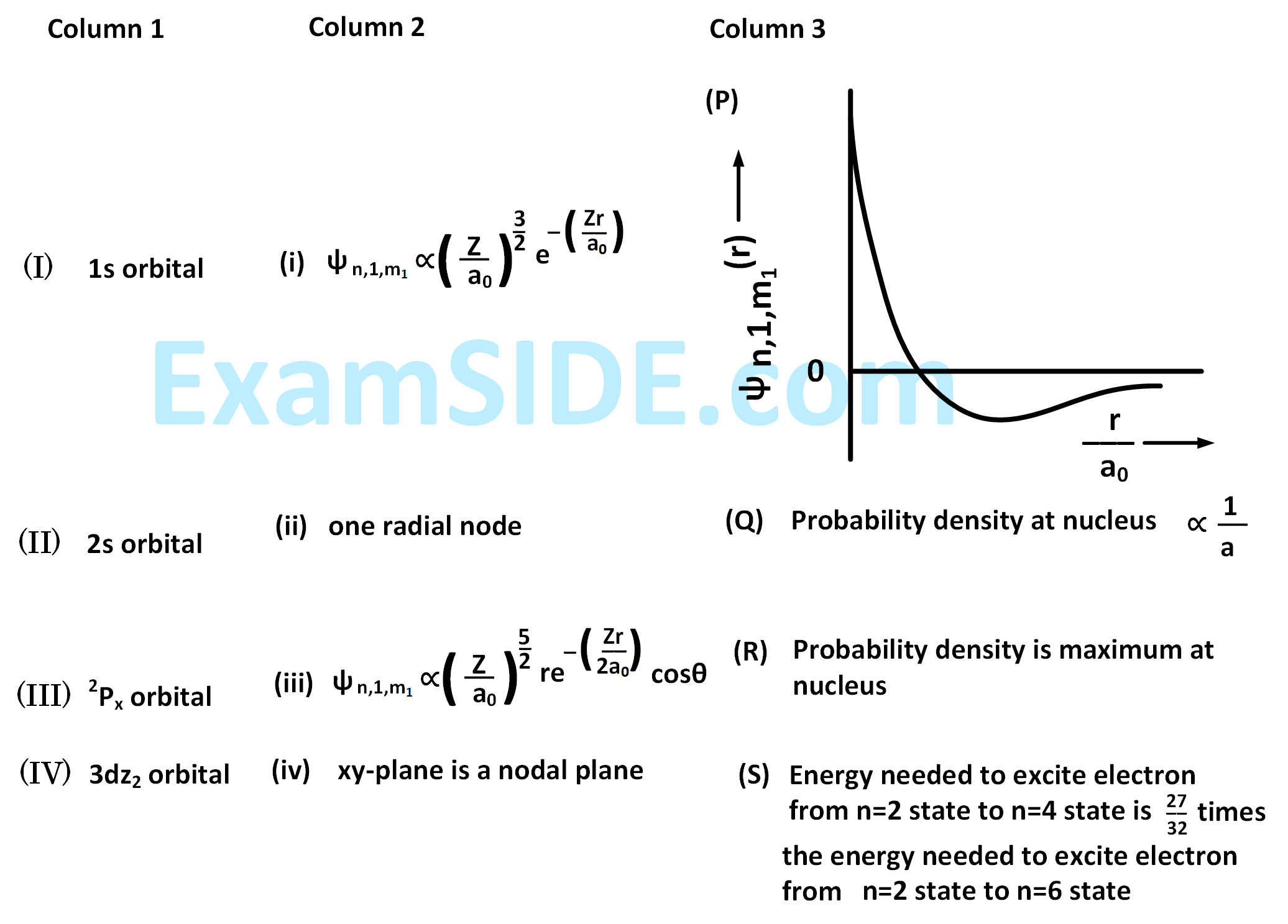

For $$H{e^ + }$$ ion, the only INCORRECT combination is
3
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1
The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.


For hydrogen atom, the only CORRECT combination is :
4
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1
The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.


For the given orbital in Column 1, the only CORRECT combination for any hydrogen-like species is :
Questions Asked from MCQ (Single Correct Answer)
JEE Advanced 2024 Paper 2 Online (1) JEE Advanced 2019 Paper 2 Offline (2) JEE Advanced 2017 Paper 1 Offline (3) JEE Advanced 2016 Paper 1 Offline (1) IIT-JEE 2012 Paper 1 Offline (1) IIT-JEE 2010 Paper 2 Offline (3) IIT-JEE 2008 Paper 2 Offline (1) IIT-JEE 2008 Paper 1 Offline (1) IIT-JEE 2006 (1) IIT-JEE 2005 Screening (1) IIT-JEE 2004 Screening (1) IIT-JEE 2003 Screening (1) IIT-JEE 2002 Screening (2) IIT-JEE 2001 Screening (2) IIT-JEE 2000 Screening (2) IIT-JEE 1999 (1) IIT-JEE 1998 (2) IIT-JEE 1997 (1) IIT-JEE 1996 (1) IIT-JEE 1995 Screening (1) IIT-JEE 1992 (2) IIT-JEE 1989 (2) IIT-JEE 1988 (3) IIT-JEE 1986 (4) IIT-JEE 1985 (3) IIT-JEE 1984 (3) IIT-JEE 1983 (3) IIT-JEE 1982 (1) IIT-JEE 1981 (1) IIT-JEE 1979 (1)
JEE Advanced Subjects
Physics
Mechanics
Electricity
Modern Physics
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Coordinate Geometry