Mechanics
Vector Algebra
MCQ (Single Correct Answer)Circular Motion
MCQ (Single Correct Answer)Simple Harmonic Motion
MCQ (Single Correct Answer)Gravitation
MCQ (Single Correct Answer)Electricity
Electromagnetic Waves
MCQ (Multiple Correct Answer)Optics
Wave Optics
MCQ (Single Correct Answer)Modern Physics
Electronic Devices
MCQ (Single Correct Answer)1
WB JEE 2022
MCQ (Single Correct Answer)
+1
-0.25
Consider a thermodynamic process where integral energy $$U = A{P^2}V$$ (A = constant). If the process is performed adiabatically, then
2
WB JEE 2022
MCQ (Single Correct Answer)
+1
-0.25
One mole of a diatomic ideal gas undergoes a process shown in P-V diagram. The total heat given to the gas (ln 2 = 0.7) is

3
WB JEE 2022
MCQ (Single Correct Answer)
+2
-0.5
One mole of an ideal monoatomic gas expands along the polytrope PV3 = constant from V1 to V2 at a constant pressure P1. The temperature during the process is such that molar specific heat $${C_V} = {{3R} \over 2}$$. The total heat absorbed during the process can be expressed as
4
WB JEE 2021
MCQ (Single Correct Answer)
+1
-0.25
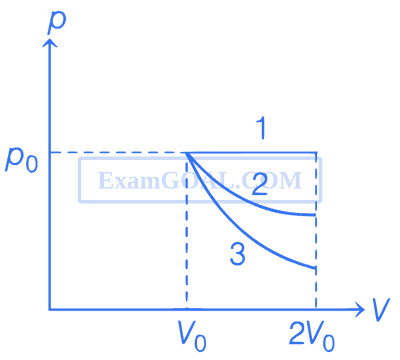
In the given figure, 1 represents isobaric, 2 represents isothermal and 3 represents adiabatic processes of an ideal gas. If $$\Delta$$U1, $$\Delta$$U2 and $$\Delta$$U3 be the changes in internal energy in these processes respectively, then
Questions Asked from MCQ (Single Correct Answer)
WB JEE Subjects
Physics
Mechanics
Electricity
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Coordinate Geometry