Mechanics
Units & Measurement and Dimensions
MCQ (Single Correct Answer)Vector Algebra
MCQ (Single Correct Answer)Motion in a Straight Line
MCQ (Single Correct Answer)Motion in a Plane
MCQ (Single Correct Answer)Circular Motion
MCQ (Single Correct Answer)Laws of Motion
MCQ (Single Correct Answer)Work, Energy and Power
MCQ (Single Correct Answer)Center of Mass and Collision
MCQ (Single Correct Answer)Rotational Motion
MCQ (Single Correct Answer)Elasticity
MCQ (Single Correct Answer)Fluid Mechanics
MCQ (Single Correct Answer)Heat and Thermodynamics
MCQ (Single Correct Answer)Simple Harmonic Motion
MCQ (Single Correct Answer)Gravitation
MCQ (Single Correct Answer)Electromagnetism
Electrostatics
MCQ (Single Correct Answer)Current Electricity
MCQ (Single Correct Answer)Capacitor
MCQ (Single Correct Answer)Moving Charges and Magnetism
MCQ (Single Correct Answer)Magnetism and Matter
MCQ (Single Correct Answer)Electromagnetic Induction
MCQ (Single Correct Answer)Alternating Current
MCQ (Single Correct Answer)Electromagnetic Waves
MCQ (Single Correct Answer)Modern Physics
Atoms and Nuclei
MCQ (Single Correct Answer)Dual Nature of Radiation
MCQ (Single Correct Answer)Semiconductor Devices and Logic Gates
MCQ (Single Correct Answer)Communication Systems
MCQ (Single Correct Answer)1
AP EAPCET 2024 - 21th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
A monoatomic gas of $n$ moles is heated from temperature $T_1$ to $T_2$ under two different conditions, (i) at constant volume and (ii) at constant pressure. The change in internal energy of the gas is
2
AP EAPCET 2024 - 21th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
In a Carnot engine, when the temperatures are $T_2=0^{\circ} \mathrm{C}$ and $T_1=200^{\circ} \mathrm{C}$, its efficiency is $\eta_1$ and when the temperature are $T_1=0^{\circ} \mathrm{C}$ and $T_2=-200^{\circ} \mathrm{C}$, its efficiency is $\eta_2$. Then, the value of $\frac{\eta_1}{\eta_2}$ is
3
AP EAPCET 2024 - 21th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
Heat energy absorbed by a system going through the cyclic process shown in the figure is
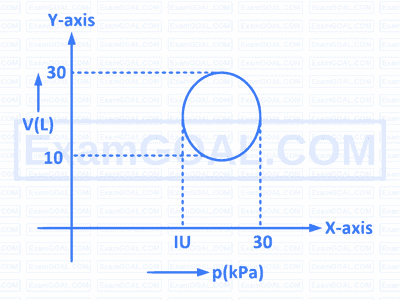
4
AP EAPCET 2024 - 21th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
A polyatomic gas with $n$ degrees of freedom has a mean kinetic energy per molecule given by (if $N$ is Avogadro's number )
Questions Asked from MCQ (Single Correct Answer)
AP EAPCET 2024 - 23th May Morning Shift (4) AP EAPCET 2024 - 22th May Evening Shift (5) AP EAPCET 2024 - 22th May Morning Shift (5) AP EAPCET 2024 - 21th May Evening Shift (5) AP EAPCET 2024 - 21th May Morning Shift (5) AP EAPCET 2024 - 20th May Evening Shift (5) AP EAPCET 2024 - 20th May Morning Shift (5) AP EAPCET 2024 - 19th May Evening Shift (5) AP EAPCET 2024 - 18th May Morning Shift (6) AP EAPCET 2022 - 5th July Morning Shift (5) AP EAPCET 2022 - 4th July Evening Shift (4) AP EAPCET 2022 - 4th July Morning Shift (4) AP EAPCET 2021 - 20th August Morning Shift (5) AP EAPCET 2021 - 19th August Evening Shift (6) AP EAPCET 2021 - 19th August Morning Shift (6)
AP EAPCET Subjects
Physics
Mechanics
Optics
Electromagnetism
Chemistry
Physical Chemistry
Inorganic Chemistry
Mathematics
Algebra
Trigonometry
Calculus
Coordinate Geometry