Thermodynamics
1
GATE ME 2017 Set 2
MCQ (Single Correct Answer)
+2
-0.6
The volume and temperature of air (assumed to be an ideal gas) in a closed vessel is $$2.87{m^3}$$ and $$300$$ $$K,$$ respectively. The gauge pressure indicated by a manometer fitted to the wall of the vessel is $$0.5$$ bar. If the gas constant of air is
$$R =287J/kgK$$ and the atmospheric pressure is $$1$$ bar, the mass of air (in $$kg$$) in the vessel is
$$R =287J/kgK$$ and the atmospheric pressure is $$1$$ bar, the mass of air (in $$kg$$) in the vessel is
2
GATE ME 2015 Set 1
Numerical
+2
-0
Temperature of nitrogen in a vessel of volume $$2{m^3}$$ is $$288$$ $$K.$$ $$A$$ $$U$$-tube manometer connected to the vessel shows a reading of $$70$$ $$cm$$ of mercury (level higher in the end open to atmosphere). The universal gas constant is $$8314$$ $$J/kmol$$-$$K,$$ atmospheric pressure is $$1.01325$$ bar, acceleration due to gravity is $$9.81$$ $$m/{s^2}$$ and density of mercury is $$13600$$ $$kg/{m^3}.$$ The mass of nitrogen (in $$kg$$) in the vessel is _________
Your input ____
3
GATE ME 2014 Set 3
MCQ (Single Correct Answer)
+2
-0.6
A certain amount of an ideal gas is initially at a pressure $${p_1}$$ and temperature $${T_1}$$. First, it undergoes a constant pressure process $$1$$-$$2$$ such that $$T{}_2 = 3{T_1}/4.$$ Then, it undergoes a constant volume process $$2$$-$$3$$ such that $${T_3} = {T_1}/2.$$ The ratio of the final volume to the initial volume of the ideal gas is
4
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
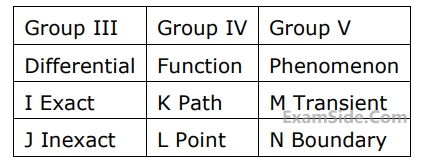
Match items from groups, $${\rm I},$$ $${\rm II},$$ $${\rm III},$$ $${\rm IV}$$ and $$V$$



Questions Asked from Marks 2
GATE ME Subjects
Engineering Mechanics
Machine Design
Strength of Materials
Heat Transfer
Production Engineering
Industrial Engineering
Turbo Machinery
Theory of Machines
Engineering Mathematics
Fluid Mechanics
Thermodynamics
General Aptitude