Thermodynamics
1
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
Match items from groups, $${\rm I},$$ $${\rm II},$$ $${\rm III},$$ $${\rm IV}$$ and $$V$$

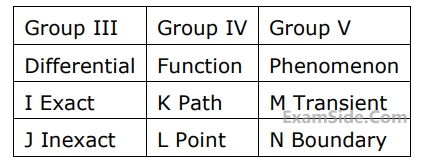


2
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
A football was inflated to a gauge pressure of $$1$$ bar when the ambient temperature was $${15^ \circ }C$$. When the game started next day, the air temperature at the stadium was $${5^ \circ }C$$.
Assume that the volume of the football remains constant at $$2500c{m^3}$$
Assume that the volume of the football remains constant at $$2500c{m^3}$$
The amount of heat lost by the air in the football and the gauge pressure of air in the football at the stadium respectively equal
3
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
A football was inflated to a gauge pressure of $$1$$ bar when the ambient temperature was $${15^ \circ }C$$. When the game started next day, the air temperature at the stadium was $${5^ \circ }C$$.
Assume that the volume of the football remains constant at $$2500c{m^3}$$
Assume that the volume of the football remains constant at $$2500c{m^3}$$
Gauge pressure of air to which the ball must have been originally inflated so that it would equal $$1$$ bar gauge at the stadium is
4
GATE ME 1999
MCQ (More than One Correct Answer)
+2
-0
An isolated thermodynamic system executes a process. Choose the correct statement $$(s)$$ from the following:
Questions Asked from Marks 2
GATE ME Subjects
Engineering Mechanics
Machine Design
Strength of Materials
Heat Transfer
Production Engineering
Industrial Engineering
Turbo Machinery
Theory of Machines
Engineering Mathematics
Fluid Mechanics
Thermodynamics
General Aptitude