Given below are two statements :
Statement (I) : Neopentane forms only one monosubstituted derivative.
Statement (II) : Melting point of neopentane is higher than n-pentane.
In the light of the above statements, choose the most appropriate answer from the options given below :
Match List - I with List - II.
| List - I (Reaction) |
List - II (Name of reaction) |
||
|---|---|---|---|
| (A) |  |
(I) | Lucas reaction |
| (B) | $$ \mathrm{ArN}_2^{+} \mathrm{X}^{-} \xrightarrow[\mathrm{HCl}]{\mathrm{Cu}} \mathrm{ArCl}+\mathrm{N}_2 \uparrow+\mathrm{CuX} $$ |
(II) | Finkelstein reaction |
| (C) | $$ \mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{NaI} \xrightarrow[\text { Acetone }]{\text { Dry }} \mathrm{C}_2 \mathrm{H}_5 \mathrm{I}+\mathrm{NaBr} $$ |
(III) | Fittig reaction |
| (D) | $$ \mathrm{CH}_3 \mathrm{C}(\mathrm{OH})\left(\mathrm{CH}_3\right) \mathrm{CH}_3 \xrightarrow[\mathrm{ZnCl}_2]{\mathrm{HCl}} \mathrm{CH}_3 \mathrm{C}(\mathrm{Cl})\left(\mathrm{CH}_3\right) \mathrm{CH}_3 $$ |
(IV) | Gatterman reaction |
$$ \text { Choose the correct answer from the options given below : } $$
Electronic configuration of four elements A, B, C and D are given below :
(A) $1 s^2 2 s^2 2 p^3$
(B) $1 s^2 2 s^2 2 p^4$
(C) $1 s^2 2 s^2 2 p^5$
(D) $1 s^2 2 s^2 2 p^2$
Which of the following is the correct order of increasing electronegativity (Pauling's scale)?
Which of the following statements are true?
(A) The subsidiary quantum number $l$ describes the shape of the orbital occupied by the electron.
(B)  is the boundary surface diagram of the $2 \mathrm{p}_x$ orbital.
is the boundary surface diagram of the $2 \mathrm{p}_x$ orbital.
(C) The + and - signs in the wave function of the $2 p_x$ orbital refer to charge.
(D) The wave function of $2 \mathrm{p}_x$ orbital is zero everywhere in the $x y$ plane.
Choose the correct answer from the options given below :
Arrange the following in order of magnitude of work done by the system/on the system at constant temperature.
(a) $\left|w_{\text {reversible }}\right|$ for expansion in infinite stages.
(b) $\left|w_{\text {irreversible }}\right|$ for expansion in single stage.
(c) $\left|\mathrm{w}_{\text {reversible }}\right|$ for compression in infinite stages.
(d) $\left|w_{\text {irreversible }}\right|$ for compression in single stage.
Choose the correct answer from the options given below :
When a concentrated solution of sulphanilic acid and 1-naphthylamine is treated with nitrous acid $(273 \mathrm{~K})$ and acidified with acetic acid, the mass $(\mathrm{g})$ of 0.1 mole of product formed is :
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{H}: 1, \mathrm{C}: 12, \mathrm{~N}: 14, \mathrm{O}: 16, \mathrm{~S}: 32$ )
Which of the following graphs correctly represents the variation of thermodynamic properties of Haber's process?
Reactant A converts to product D through the given mechanism (with the net evolution of heat):
A → B slow; ΔH = +ve
B → C fast; ΔH = -ve
C → D fast; ΔH = -ve
Which of the following represents the above reaction mechanism?
Consider the following reactions. From these reactions which reaction will give carboxylic acid as a major product ?
(A) $\quad \mathrm{R}-\mathrm{C} \equiv \mathrm{N} \xrightarrow[\text { mild condition }]{\text { (i) } \stackrel{+}{\mathrm{H}} / \mathrm{H}_2 \mathrm{O}}$
(B) $\quad \mathrm{R}-\mathrm{MgX} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{CO}_2}$
(C) $\mathrm{R}-\mathrm{C} \equiv \mathrm{N} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{SnCl}_2 / \mathrm{HCl}}$
(D) $\quad \mathrm{R} \cdot \mathrm{CH}_2 \cdot \mathrm{OH} \xrightarrow{\mathrm{PCC}}$
(E) 
Choose the correct answer from the options given below :
' $x$ ' g of NaCl is added to water in a beaker with a lid. The temperature of the system is raised from $1^{\circ} \mathrm{C}$ to $25^{\circ} \mathrm{C}$. Which out of the following plots, is best suited for the change in the molarity $(\mathrm{M})$ of the solution with respect to temperature ?
[Consider the solubility of NaCl remains unchanged over the temperature range]
$$ \text { Match List - I with List - II. } $$
| List - I (Purification technique) |
List - II (Mixture of organic compounds) |
||
|---|---|---|---|
| (A) | $$ \text { Distillation (simple) } $$ |
(I) | Diesel + Petrol |
| (B) | $$ \text { Fractional distillation } $$ |
(II) | Aniline + Water |
| (C) | $$ \text { Distillation under reduced pressure } $$ |
(III) | Chloroform + Aniline |
| (D) | $$ \text { Steam distillation } $$ |
(IV) | Glycerol + Spent-lye |
$$ \text { Choose the correct answer from the options given below : } $$
Consider the following chemical equilibrium of the gas phase reaction at a constant temperature : $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{B}(\mathrm{g})+\mathrm{C}(\mathrm{g})$
If $p$ being the total pressure, $K_p$ is the pressure equilibrium constant and $\alpha$ is the degree of dissociation, then which of the following is true at equilibrium?
For the reaction $\mathrm{A} \rightarrow \mathrm{B}$ the following graph was obtained. The time required (in seconds) for the concentration of A to reduce to $2.5 \mathrm{~g} \mathrm{~L}^{-1}$ (if the initial concentration of A was $50 \mathrm{~g} \mathrm{~L}^{-1}$ ) is $\qquad$ . (Nearest integer)
Given : $\log 2=0.3010$

When 1 g each of compounds AB and $\mathrm{AB}_2$ are dissolved in 15 g of water separately, they increased the boiling point of water by 2.7 K and 1.5 K respectively. The atomic mass of A (in $a m u$ ) is____________ $\times 10^{-1}$ (Nearest integer)
(Given : Molal boiling point elevation constant is $0.5 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ )
The spin-only magnetic moment value of $\mathrm{M}^{\mathrm{n}+}$ ion formed among $\mathrm{Ni}, \mathrm{Zn}, \mathrm{Mn}$ and Cu that has the least enthalpy of atomisation is_________ . (in nearest integer) Here n is equal to the number of diamagnetic complexes among $\mathrm{K}_2\left[\mathrm{NiCl}_4\right],\left[\mathrm{Zn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2$, $\mathrm{K}_3\left[\mathrm{Mn}(\mathrm{CN})_6\right]$ and $\left[\mathrm{Cu}\left(\mathrm{PPh}_3\right)_3 \mathrm{I}\right]$
$0.2 \%(\mathrm{w} / \mathrm{v})$ solution of NaOH is measured to have resistivity $870.0 \mathrm{~m} \Omega \mathrm{~m}$. The molar conductivity of the solution will be__________$\times 10^2 \mathrm{mS} \mathrm{dm}^2 \mathrm{~mol}^{-1}$. (Nearest integer)

Consider the above sequence of reactions. 151 g of 2-bromopentane is made to react. Yield of major product P is $80 \%$ whereas Q is $100 \%$.
Mass of product Q obtained is________ g.
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{H}: 1, \mathrm{C}: 12, \mathrm{O}: 16, \mathrm{Br}: 80$ )
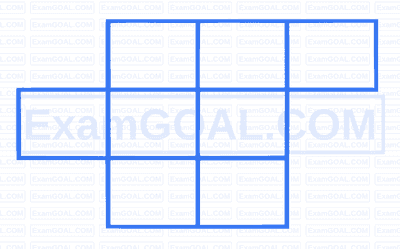
If the system of equations
$$ \begin{aligned} & 2 x+\lambda y+3 z=5 \\ & 3 x+2 y-z=7 \\ & 4 x+5 y+\mu z=9 \end{aligned} $$
has infinitely many solutions, then $\left(\lambda^2+\mu^2\right)$ is equal to :
$$ \text { Given three indentical bags each containing } 10 \text { balls, whose colours are as follows : } $$
$$ \begin{array}{lccc} & \text { Red } & \text { Blue } & \text { Green } \\ \text { Bag I } & 3 & 2 & 5 \\ \text { Bag II } & 4 & 3 & 3 \\ \text { Bag III } & 5 & 1 & 4 \end{array} $$
A person chooses a bag at random and takes out a ball. If the ball is Red, the probability that it is from bag I is p and if the ball is Green, the probability that it is from bag III is $q$, then the value of $\left(\frac{1}{p}+\frac{1}{q}\right)$ is:$$ \text { If } y=\cos \left(\frac{\pi}{3}+\cos ^{-1} \frac{x}{2}\right) \text {, then }(x-y)^2+3 y^2 \text { is equal to } $$
If the set of all $\mathrm{a} \in \mathbf{R}-\{1\}$, for which the roots of the equation $(1-\mathrm{a}) x^2+2(\mathrm{a}-3) x+9=0$ are positive is $(-\infty,-\alpha] \cup[\beta, \gamma)$, then $2 \alpha+\beta+\gamma$ is equal to $\qquad$ .
In a moving coil galvanometer, two moving coils $\mathrm{M}_1$ and $\mathrm{M}_2$ have the following particulars :
$$ \begin{aligned} & \mathrm{R}_1=5 \Omega, \mathrm{~N}_1=15, \mathrm{~A}_1=3.6 \times 10^{-3} \mathrm{~m}^2, \mathrm{~B}_1=0.25 \mathrm{~T} \\ & \mathrm{R}_2=7 \Omega, \mathrm{~N}_2=21, \mathrm{~A}_2=1.8 \times 10^{-3} \mathrm{~m}^2, \mathrm{~B}_2=0.50 \mathrm{~T} \end{aligned} $$
Assuming that torsional constant of the springs are same for both coils, what will be the ratio of voltage sensitivity of $M_1$ and $M_2$ ?
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Net dipole moment of a polar linear isotropic dielectric substance is not zero even in the absence of an external electric field.
Reason (R) : In absence of an external electric field, the different permanent dipoles of a polar dielectric substance are oriented in random directions.
In the light of the above statements, choose the most appropriate answer from the options given below :

$$ \text { In the digital circuit shown in the figure, for the given inputs the } P \text { and } Q \text { values are : } $$

An electron with mass ' m ' with an initial velocity $(\mathrm{t}=0) \overrightarrow{\mathrm{v}}=\mathrm{v}_0 \hat{i}\left(\mathrm{v}_0>0\right)$ enters a magnetic field $\overrightarrow{\mathrm{B}}=\mathrm{B}_0 \hat{j}$. If the initial de-Broglie wavelength at $\mathrm{t}=0$ is $\lambda_0$ then its value after time ' t ' would be :

$$ \begin{array}{lll} & \text { List - I } & {List - II }\\ \text { } \\ \text { (A) } & \text { Heat capacity of body } & \text { (I) } \mathrm{J} \mathrm{~kg}^{-1} \\ \text { (B) } & \text { Specific heat capacity of body } & \text { (II) } \mathrm{J} \mathrm{~K}^{-1} \\ \text { (C) } & \text { Latent heat } & \text { (III) } \mathrm{J} \mathrm{~kg}^{-1} \mathrm{~K}^{-1} \\ \text { (D) } & \text { Thermal conductivity } & \text { (IV) } \mathrm{J} \mathrm{~m}^{-1} \mathrm{~K}^{-1} \mathrm{~s}^{-1} \end{array} $$
$$ \text { Choose the correct answer from the options given below : } $$
A body of mass 1 kg is suspended with the help of two strings making angles as shown in figure. Magnitudes of tensions $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively, are (in N ) :
(Take acceleration due to gravity $10 \mathrm{~m} / \mathrm{s}^2$ )

A wheel of radius 0.2 m rotates freely about its center when a string that is wrapped over its rim is pulled by force of 10 N as shown in figure. The established torque produces an angular acceleration of $2 \mathrm{rad} / \mathrm{s}^2$. Moment of intertia of the wheel is___________ $\mathrm{kg} \mathrm{}\,\, \mathrm{m}^2$. (Acceleration due to gravity $=10 \mathrm{~m} / \mathrm{s}^2$ )
The internal energy of air in $4 \mathrm{~m} \times 4 \mathrm{~m} \times 3 \mathrm{~m}$ sized room at 1 atmospheric pressure will be___________________$\times 10^6 \mathrm{~J}$
(Consider air as diatomic molecule)
A satellite of mass 1000 kg is launched to revolve around the earth in an orbit at a height of 270 km from the earth's surface. Kinetic energy of the satellite in this orbit is____________ $\times 10^{10} \mathrm{~J}$.
(Mass of earth $=6 \times 10^{24} \mathrm{~kg}$, Radius of earth $=6.4 \times 10^6 \mathrm{~m}$, Gravitational constant $=6.67 \times 10^{-11} \mathrm{Nm}^2 \mathrm{~kg}^{-2}$ )