Chemistry
1
For the given reaction

What is 'A'?

What is 'A'?
2
Which of the following is 'a' FALSE statement ?
3
Find A, B and C in the following reactions :
$$N{H_3} + A + C{O_2} \to {(N{H_4})_2}C{O_3}$$
$${(N{H_4})_2}C{O_3} + {H_2}O + B \to N{H_4}HC{O_3}$$
$$N{H_4}HC{O_3} + NaCl \to N{H_4}Cl + C$$
$$N{H_3} + A + C{O_2} \to {(N{H_4})_2}C{O_3}$$
$${(N{H_4})_2}C{O_3} + {H_2}O + B \to N{H_4}HC{O_3}$$
$$N{H_4}HC{O_3} + NaCl \to N{H_4}Cl + C$$
4
Match List - I with List - II.
Choose the most appropriate answer from the options given below :
| List I Electronic configuration |
List II $${\Delta _i}H$$ in kJ $$mo{l^{ - 1}}$$ |
||
|---|---|---|---|
| (a) | $$1{s^2}2{s^2}$$ | (i) | 801 |
| (b) | $$1{s^2}2{s^2}2{p^4}$$ | (ii) | 899 |
| (c) | $$1{s^2}2{s^2}2{p^3}$$ | (iii) | 1314 |
| (d) | $$1{s^2}2{s^2}2{p^1}$$ | (iv) | 1402 |
Choose the most appropriate answer from the options given below :
5
Given below are two statements :
Statement I : A mixture of chloroform and aniline can be separated by simple distillation.
Statement II : When separating aniline from a mixture of aniline and water by steam distillation aniline boils below its boiling point.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : A mixture of chloroform and aniline can be separated by simple distillation.
Statement II : When separating aniline from a mixture of aniline and water by steam distillation aniline boils below its boiling point.
In the light of the above statements, choose the most appropriate answer from the options given below :
6
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason R : Fluorine is the most electronegative element and hydrogen bonds in HF are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below :
Assertion A : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason R : Fluorine is the most electronegative element and hydrogen bonds in HF are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below :
7
Which one of the following lanthanoids does not form MO2? [M is lanthanoid metal]
8
For the given reaction :

What is 'A'?

What is 'A'?
9
Compound A used as a strong oxidizing agent is amphoteric in nature. It is the part of lead storage batteries. Compound A is :
10
Identify the major products A and B respectively in the following reactions of phenol :


11
An amine on reaction with benzensulphonyl chloride produces a compound insoluble in alkaline solution. This amine can be prepared by ammonolysis of ethyl chloride. The correct structure of amine is :
12
Given below are two statements :
Statement I : o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement II : o-Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement II : o-Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :
13

B reacts with Hydroxyl amine but does not give Tollen's test. Identify A and B.
14
Which of the following vitamin is helpful in delaying the blood clotting?
15
The orbital having two radial as well as two angular nodes is :
16
For a chemical reaction A + B ⇌ C + D
($${\Delta _r}{H^\Theta }$$ = 80 kJ mol$$-$$1) the entropy change $${\Delta _r}{S^\Theta }$$ depends on the temperature T (in K) as $${\Delta _r}{S^\Theta }$$ = 2T (J K$$-$$1mol$$-$$1).
Minimum temperature at which it will become spontaneous is ___________ K. (Integer)
($${\Delta _r}{H^\Theta }$$ = 80 kJ mol$$-$$1) the entropy change $${\Delta _r}{S^\Theta }$$ depends on the temperature T (in K) as $${\Delta _r}{S^\Theta }$$ = 2T (J K$$-$$1mol$$-$$1).
Minimum temperature at which it will become spontaneous is ___________ K. (Integer)
17
An exothermic reaction X $$ \to $$ Y has an activation energy 30 kJ mol$$-$$1. If energy change $$\Delta$$E during the reaction is $$-$$20 kJ, then the activation energy for the reverse reaction in kJ is ___________. (Integer answer)
18
Dichromate ion is treated with base, the oxidation number of Cr in the product formed is ___________.
19
Number of bridging CO ligands in [Mn2(CO)10] is __________.
20
Consider the following reaction
$$MnO_4^ - + 8{H^ + } + 5{e^ - } \to M{n^{ + 2}} + 4{H_2}O,{E^o} = 1.51V$$.
The quantity of electricity required in Faraday to reduce five moles of $$MnO_4^ - $$ is ___________. (Integer answer)
$$MnO_4^ - + 8{H^ + } + 5{e^ - } \to M{n^{ + 2}} + 4{H_2}O,{E^o} = 1.51V$$.
The quantity of electricity required in Faraday to reduce five moles of $$MnO_4^ - $$ is ___________. (Integer answer)
21
224 mL of SO2(g) at 298 K and 1 atm is passed through 100 mL of 0.1 M NaOH solution. The non-volatile solute produced is dissolved in 36g of water. The lowering of vapour pressure of solution (assuming the solution in dilute) (P$$_{({H_2}O)}^o$$ $$-$$ 24 mm of Hg) is x $$\times$$ 10$$-$$2 mm of Hg, the value of x is ___________. (Integer answer)
22
A homogeneous ideal gaseous reaction $$A{B_{2(g)}} \rightleftharpoons {A_{(g)}} + 2{B_{(g)}}$$ is carried out in a 25 litre flask at 27$$^\circ$$C. The initial amount of AB2 was 1 mole and the equilibrium pressure was 1.9 atm. The value of Kp is x $$\times$$ 10$$-$$2. The value of x is _________. (Integer answer)
[R = 0.08206 dm3atm K$$-$$1mol$$-$$1]
[R = 0.08206 dm3atm K$$-$$1mol$$-$$1]
Mathematics
1
The maximum value of the term independent of 't' in the expansion
of $${\left( {t{x^{{1 \over 5}}} + {{{{(1 - x)}^{{1 \over {10}}}}} \over t}} \right)^{10}}$$ where x$$\in$$(0, 1) is :
of $${\left( {t{x^{{1 \over 5}}} + {{{{(1 - x)}^{{1 \over {10}}}}} \over t}} \right)^{10}}$$ where x$$\in$$(0, 1) is :
2
The value of $$\mathop {\lim }\limits_{h \to 0} 2\left\{ {{{\sqrt 3 \sin \left( {{\pi \over 6} + h} \right) - \cos \left( {{\pi \over 6} + h} \right)} \over {\sqrt 3 h\left( {\sqrt 3 \cosh - \sinh } \right)}}} \right\}$$ is :
3
Let A be a symmetric matrix of order 2 with integer entries. If the sum of the diagonal elements of A2 is 1, then the possible number of such matrices is :
4
The value of $$\int\limits_{ - \pi /2}^{\pi /2} {{{{{\cos }^2}x} \over {1 + {3^x}}}} dx$$ is :
5
The number of seven digit integers with sum of the digits equal to 10 and formed by using the digits 1, 2 and 3 only is :
6
Let R = {(P, Q) | P and Q are at the same distance from the origin} be a relation, then the equivalence class of (1, $$-$$1) is the set :
7
The value of $$\sum\limits_{n = 1}^{100} {\int\limits_{n - 1}^n {{e^{x - [x]}}dx} } $$, where [ x ] is the greatest integer $$ \le $$ x, is :
8
The intersection of three lines x $$-$$ y = 0, x + 2y = 3 and 2x + y = 6 is a :
9
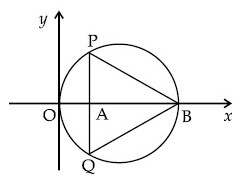
In the circle given below, let OA = 1 unit, OB = 13 unit and PQ $$ \bot $$ OB. Then, the area of the triangle PQB (in square units) is :

10
The value of $$\left| {\matrix{
{(a + 1)(a + 2)} & {a + 2} & 1 \cr
{(a + 2)(a + 3)} & {a + 3} & 1 \cr
{(a + 3)(a + 4)} & {a + 4} & 1 \cr
} } \right|$$ is :
11
If $${{{{\sin }^1}x} \over a} = {{{{\cos }^{ - 1}}x} \over b} = {{{{\tan }^{ - 1}}y} \over c}$$; $$0 < x < 1$$,
then the value of $$\cos \left( {{{\pi c} \over {a + b}}} \right)$$ is :
then the value of $$\cos \left( {{{\pi c} \over {a + b}}} \right)$$ is :
12
The maximum slope of the curve $$y = {1 \over 2}{x^4} - 5{x^3} + 18{x^2} - 19x$$ occurs at the point :
13
The rate of growth of bacteria in a culture is proportional to the number of bacteria present and the bacteria count is 1000 at initial time t = 0. The number of bacteria is increased by 20% in 2 hours. If the population of bacteria is 2000 after $${k \over {{{\log }_e}\left( {{6 \over 5}} \right)}}$$ hours, then $${\left( {{k \over {{{\log }_e}2}}} \right)^2}$$ is equal to :
14
In an increasing geometric series, the sum of the second and the sixth term is $${{25} \over 2}$$ and the product of the third and fifth term is 25. Then, the sum of 4th, 6th and 8th terms is equal to :
15
The value of the integral $$\int\limits_0^\pi {|{{\sin }\,}2x|dx} $$ is ___________.
16
The number of solutions of the equation log4(x $$-$$ 1) = log2(x $$-$$ 3) is _________.
17
The sum of 162th power of the roots of the equation x3 $$-$$ 2x2 + 2x $$-$$ 1 = 0 is ________.
18
The area bounded by the lines y = || x $$-$$ 1 | $$-$$ 2 | is ___________.
19
The difference between degree and order of a differential equation that represents the family of curves given by $${y^2} = a\left( {x + {{\sqrt a } \over 2}} \right)$$, a > 0 is _________.
20
If y = y(x) is the solution of the equation
$${e^{\sin y}}\cos y{{dy} \over {dx}} + {e^{\sin y}}\cos x = \cos x$$, y(0) = 0; then
$$1 + y\left( {{\pi \over 6}} \right) + {{\sqrt 3 } \over 2}y\left( {{\pi \over 3}} \right) + {1 \over {\sqrt 2 }}y\left( {{\pi \over 4}} \right)$$ is equal to ____________.
$${e^{\sin y}}\cos y{{dy} \over {dx}} + {e^{\sin y}}\cos x = \cos x$$, y(0) = 0; then
$$1 + y\left( {{\pi \over 6}} \right) + {{\sqrt 3 } \over 2}y\left( {{\pi \over 3}} \right) + {1 \over {\sqrt 2 }}y\left( {{\pi \over 4}} \right)$$ is equal to ____________.
21
The number of integral values of 'k' for which the equation $$3\sin x + 4\cos x = k + 1$$ has a solution, k$$\in$$R is ___________.
Physics
1
Consider the combination of 2 capacitors C1 and C2 with C2 > C1, when connected in parallel, the equivalent capacitance is $${{15} \over 4}$$ times the equivalent capacitance of the same connected in series. Calculate the ratio of capacitors, $${{{C_2}} \over {{C_1}}}$$.
2
An alternating current is given by the equation i = i1 sin $$\omega$$t + i2 cos $$\omega$$t. The rms current will be :
3
Four identical solid spheres each of mass 'm' and radius 'a' are placed with their centres on the four corners of a square of side 'b'. The moment of inertia of the system about one side of square where the axis of rotation is parallel to the plane of the square is :
4
A large number of water drops, each of radius r, combine to have a drop of radius R. If the surface tension is T and mechanical equivalent of heat is J, the rise in heat energy per unit volume will be :
5
LED is constructed from Ga-As-P semiconducting material. The energy gap of this LED is 1.9 eV. Calculate the wavelength of light emitted and its colour.
[h = 6.63 $$\times$$ 10$$-$$34 Js and c = 3 $$\times$$ 108 ms$$-$$1]
[h = 6.63 $$\times$$ 10$$-$$34 Js and c = 3 $$\times$$ 108 ms$$-$$1]
6
A short straight object of height 100 cm lies before the central axis of a spherical mirror whose focal length has absolute value | f | = 40 cm. The image of object produced by the mirror is of height 25 cm and has the same orientation of the object. One may conclude from the information :
7
In a Young's double slit experiment two slits are separated by 2 mm and the screen is placed one meter away. When a light of wavelength 500 nm is used, the fringe separation will be :
8
Assume that a tunnel is dug along a chord of the earth, at a perpendicular distance (R/2) from the earth's centre, where 'R' is the radius of the Earth. The wall of the tunnel is frictionless. If a particle is released in this tunnel, it will execute a simple harmonic motion with a time period :
9
In a typical combustion engine the workdone by a gas molecule is given by $$W = {\alpha ^2}\beta {e^{{{ - \beta {x^2}} \over {kT}}}}$$, where x is the displacement, k is the Boltzmann constant and T is the temperature. If $$\alpha$$ and $$\beta$$ are constants, dimensions of $$\alpha$$ will be :
10
If two similar springs each of spring constant K1 are joined in series, the new spring constant and time period would be changed by a factor :
11
A particle is moving with uniform speed along the circumference of a circle of radius R under the action of a central fictitious force F which is inversely proportional to R3. Its time period of revolution will be given by :
12
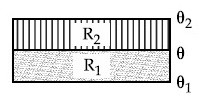
The temperature $$\theta$$ at the junction of two insulating sheets, having thermal resistances R1 and R2 as well as top and bottom temperatures $$\theta$$1 and $$\theta$$2 (as shown in figure) is given by :

13
Find the electric field at point P (as shown in figure) on the perpendicular bisector of a uniformly charged thin wire of length L carrying a charge Q. The distance of the point P from the centre of the rod is a = $${{\sqrt 3 } \over 2}L$$.


14
A planet revolving in elliptical orbit has :
A. a constant velocity of revolution.
B. has the least velocity when it is nearest to the sun.
C. its areal velocity is directly proportional to its velocity.
D. areal velocity is inversely proportional to its velocity.
E. to follow a trajectory such that the areal velocity is constant.
Choose the correct answer from the options given below :
A. a constant velocity of revolution.
B. has the least velocity when it is nearest to the sun.
C. its areal velocity is directly proportional to its velocity.
D. areal velocity is inversely proportional to its velocity.
E. to follow a trajectory such that the areal velocity is constant.
Choose the correct answer from the options given below :
15
Given below are two statements : one is labeled as Assertion A and the other is labelled as Reason R.
Assertion A : An electron microscope can achieve better resolving power than an optical microscope.
Reason R : The de Broglie's wavelength of the electrons emitted from an electron gun is much less than wavelength of visible light.
In the light of the above statements, choose the correct answer from the options given below :
Assertion A : An electron microscope can achieve better resolving power than an optical microscope.
Reason R : The de Broglie's wavelength of the electrons emitted from an electron gun is much less than wavelength of visible light.
In the light of the above statements, choose the correct answer from the options given below :
16
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Body 'P' having mass M moving with speed 'u' has head-on collision elastically with another body 'Q' having mass 'm' initially at rest. If m << M, body 'Q' will have a maximum speed equal to '2u' after collision.
Reason R : During elastic collision, the momentum and kinetic energy are both conserved.
In the light of the above statements, choose the most appropriate answer from the options given below :
Assertion A : Body 'P' having mass M moving with speed 'u' has head-on collision elastically with another body 'Q' having mass 'm' initially at rest. If m << M, body 'Q' will have a maximum speed equal to '2u' after collision.
Reason R : During elastic collision, the momentum and kinetic energy are both conserved.
In the light of the above statements, choose the most appropriate answer from the options given below :
17
The normal density of a material is $$\rho$$ and its bulk modulus of elasticity is K. The magnitude of increase in density of material, when a pressure P is applied uniformly on all sides, will be :
18
If $$\lambda$$1 and $$\lambda$$2 are the wavelengths of the third member of Lyman and first member of the Paschen series respectively, then the value of $$\lambda$$1 : $$\lambda$$2 is :
19
Find the gravitational force of attraction between the ring and sphere as shown in the diagram, where the plane of the ring is perpendicular to the line joining the centres. If $$\sqrt 8 $$R is the distance between the centres of a ring (of mass 'm') and a sphere (mass 'M') where both have equal radius 'R'.


20
Five equal resistances are connected in a network as shown in figure. The net resistance between the points A and B is :


21
A person standing on a spring balance inside a stationary lift measures 60 kg. The weight of that person if the lift descends with uniform downward acceleration of 1.8 m/s2 will be ______________ N. [g = 10 m/s2]
22
The circuit contains two diodes each with a forward resistance of 50$$\Omega$$ and with infinite reverse resistance. If the battery voltage is 6V, the current through the 120$$\Omega$$ resistance is ____________ mA.


23
In a series LCR resonant circuit, the quality factor is measured as 100. If the inductance is increased by two fold and resistance is decreased by two fold, then the quality factor after this change will be __________.
24
The mass per unit length of a uniform wire is 0.135 g/cm. A transverse wave of the form y = $$-$$ 0.21 sin (x + 30t) is produced in it, where x is in meter and t is in second. Then, the expected value of tension in the wire is x $$\times$$ 10$$-$$2 N. Value of x is _________. (Round off to the nearest integer)
25
In an electrical circuit, a battery is connected to pass 20C of charge through it in a certain given time. The potential difference between two plates of the battery is maintained at 15V. The workdone by the battery is __________J.
26
A boy pushes a box of mass 2 kg with a force $$\overrightarrow F = \left( {20\widehat i + 10\widehat j} \right)N$$ on a frictionless surface. If the box was initially at rest, then ___________ m is displacement along the x-axis after 10s.
27
A radiation is emitted by 1000W bulb and it generates an electric field and magnetic field at P, placed at a distance of 2m. The efficiency of the bulb is 1.25%. The value of peak electric field at P is x $$\times$$ 10$$-$$1 V/m. Value of x is ___________. (Rounded off to the nearest integer) [Take $${\varepsilon _0} = 8.85 \times {10^{ - 12}}$$ C2N$$-$$1 m$$-$$2, c = $$3 \times {10^8}$$ ms$$-$$1]
28
A container is divided into two chambers by a partition. The volume of first chamber is 4.5 litre and second chamber is 5.5 litre. The first chamber contain 3.0 moles of gas at pressure 2.0 atm and second chamber contain 4.0 moles of gas at pressure 3.0 atm. After the partition is removed and the mixture attains equilibrium, then, the common equilibrium pressure existing in the mixture is x $$\times$$ 10$$-$$1 atm. Value of x is ________.
29
As shown in the figure, a block of mass $$\sqrt 3 $$ kg is kept on a horizontal rough surface of coefficient of friction $${1 \over {3\sqrt 3 }}$$. The critical force to be applied on the vertical surface as shown at an angle 60$$^\circ$$ with horizontal such that it does not move, will be 3x. The value of x will be _________.
[g = 10 m/s2; sin60$$^\circ$$ = $${{\sqrt 3 } \over 2}$$; cos60$$^\circ$$ = $${1 \over 2}$$]
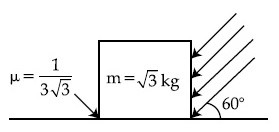
[g = 10 m/s2; sin60$$^\circ$$ = $${{\sqrt 3 } \over 2}$$; cos60$$^\circ$$ = $${1 \over 2}$$]

1
JEE Main 2021 (Online) 26th February Morning Shift
Numerical
+4
-1
In an electrical circuit, a battery is connected to pass 20C of charge through it in a certain given time. The potential difference between two plates of the battery is maintained at 15V. The workdone by the battery is __________J.
Your input ____
2
JEE Main 2021 (Online) 26th February Morning Shift
Numerical
+4
-1
A boy pushes a box of mass 2 kg with a force $$\overrightarrow F = \left( {20\widehat i + 10\widehat j} \right)N$$ on a frictionless surface. If the box was initially at rest, then ___________ m is displacement along the x-axis after 10s.
Your input ____
3
JEE Main 2021 (Online) 26th February Morning Shift
Numerical
+4
-1
A radiation is emitted by 1000W bulb and it generates an electric field and magnetic field at P, placed at a distance of 2m. The efficiency of the bulb is 1.25%. The value of peak electric field at P is x $$\times$$ 10$$-$$1 V/m. Value of x is ___________. (Rounded off to the nearest integer) [Take $${\varepsilon _0} = 8.85 \times {10^{ - 12}}$$ C2N$$-$$1 m$$-$$2, c = $$3 \times {10^8}$$ ms$$-$$1]
Your input ____
4
JEE Main 2021 (Online) 26th February Morning Shift
Numerical
+4
-1
A container is divided into two chambers by a partition. The volume of first chamber is 4.5 litre and second chamber is 5.5 litre. The first chamber contain 3.0 moles of gas at pressure 2.0 atm and second chamber contain 4.0 moles of gas at pressure 3.0 atm. After the partition is removed and the mixture attains equilibrium, then, the common equilibrium pressure existing in the mixture is x $$\times$$ 10$$-$$1 atm. Value of x is ________.
Your input ____
Subject
Chemistry
22
Mathematics
21
Physics
29
More Papers of JEE Main
2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002