Chemistry
1
Which one of the following complexes is violet in colour?
2
Which one of the following when dissolved in water gives coloured solution in nitrogen atmosphere?
3
The major products formed in the following reaction sequence A and B are :


4
The major product formed in the following reaction is :


5
The major product formed in the following reaction is :


6
Given below are two statements :
Statement I : The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below :
7
The correct options for the products A and B of the following reactions are :


8
Given below are two statements.
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with NaOH phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with NaOH phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below :
9
Among the following compounds I-IV, which one forms a yellow precipitate on reacting sequentially with (i) NaOH (ii) dil. HNO3 (iii) AgNO3?


10
Given below are two statements.
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below :
11
The correct sequential addition of reagents in the preparation of 3-nitrobenzoic acid from benzene is :
12
Excess of isobutane on reaction with Br2 in presence of light at 125$$^\circ$$C gives which one of the following, as the major product?
13
AB3 is an interhalogen T-shaped molecule. The number of lone pairs of electrons on A is __________. (Integer answer)
14
These are physical properties of an element
(A) Sublimation enthalpy
(B) Ionisation enthalpy
(C) Hydration enthalpy
(D) Electron gain enthalpy
The total number of above properties that affect the reduction potential is ____________ (Integer answer)
(A) Sublimation enthalpy
(B) Ionisation enthalpy
(C) Hydration enthalpy
(D) Electron gain enthalpy
The total number of above properties that affect the reduction potential is ____________ (Integer answer)
15
Of the following four aqueous solutions, total number of those solutions whose freezing point is lower than that of 0.10 M C2H5OH is __________ (Integer answer)
(i) 0.10 M Ba3(PO4)2
(ii) 0.10 M Na2SO4
(iii) 0.10 M KCl
(iv) 0.10 M Li3PO4
(i) 0.10 M Ba3(PO4)2
(ii) 0.10 M Na2SO4
(iii) 0.10 M KCl
(iv) 0.10 M Li3PO4
16
The OH$$-$$ concentration in a mixture of 5.0 mL of 0.0504 M NH4Cl and 2 mL of 0.0210 M NH3 solution is x $$\times$$ 10$$-$$6 M. The value of x is ___________. (Nearest integer)
[Given Kw = 1 $$\times$$ 10$$-$$14 and Kb = 1.8 $$\times$$ 10$$-$$5]
[Given Kw = 1 $$\times$$ 10$$-$$14 and Kb = 1.8 $$\times$$ 10$$-$$5]
17
The number of 4f electrons in the ground statement electronic configuration of Gd2+ is ___________. [Atomic number of Gd = 64]
18
The ratio of number of water molecules in Mohr's salt and potash alum is ____________ $$\times$$ 10$$-$$1. (Integer answer)
19
The following data was obtained for chemical reaction given below at 975 K.
2NO(g) + 2H2(g) $$\to$$ N2(g) + 2H2O(g)

The order of the reaction with respect to NO is ___________. [Integer answer]
2NO(g) + 2H2(g) $$\to$$ N2(g) + 2H2O(g)

The order of the reaction with respect to NO is ___________. [Integer answer]
20
The Born-Haber cycle for KCl is evaluated with the following data :
$${\Delta _f}{H^\Theta }$$ for KCl = $$-$$436.7 kJ mol$$-$$1 ;
$${\Delta _{sub}}{H^\Theta }$$ for K = 89.2 kJ mol$$-$$1 ;
$${\Delta _{ionization}}{H^\Theta }$$ for K = 419.0 kJ mol$$-$$1 ;
$${\Delta _{electron\,gain}}{H^\Theta }$$ for Cl(g) = $$-$$348.6 kJ mol$$-$$1 ;
$${\Delta _{bond}}{H^\Theta }$$ for Cl2 = 243.0 kJ mol$$-$$1
The magnitude of lattice enthalpy of KCl in kJ mol$$-$$1 is _____________ (Nearest integer)
$${\Delta _f}{H^\Theta }$$ for KCl = $$-$$436.7 kJ mol$$-$$1 ;
$${\Delta _{sub}}{H^\Theta }$$ for K = 89.2 kJ mol$$-$$1 ;
$${\Delta _{ionization}}{H^\Theta }$$ for K = 419.0 kJ mol$$-$$1 ;
$${\Delta _{electron\,gain}}{H^\Theta }$$ for Cl(g) = $$-$$348.6 kJ mol$$-$$1 ;
$${\Delta _{bond}}{H^\Theta }$$ for Cl2 = 243.0 kJ mol$$-$$1
The magnitude of lattice enthalpy of KCl in kJ mol$$-$$1 is _____________ (Nearest integer)
21
The total number of negative charge in the tetrapeptide, Gly-Glu-Asp-Tyr, at pH 12.5 will be ______________. (Integer answer)
22
An aqueous KCl solution of density 1.20 g mL$$-$$1 has a molality of 3.30 mol kg$$-$$1. The molarity of the solution in mol L$$-$$1 is ____________ (Nearest integer) [Molar mass of KCl = 74.5]
Mathematics
1
The mean and standard deviation of 20 observations were calculated as 10 and 2.5 respectively. It was found that by mistake one data value was taken as 25 instead of 35. if $$\alpha$$ and $$\sqrt \beta $$ are the mean and standard deviation respectively for correct data, then ($$\alpha$$, $$\beta$$) is :
2
Let y = y(x) be a solution curve of the differential equation $$(y + 1){\tan ^2}x\,dx + \tan x\,dy + y\,dx = 0$$, $$x \in \left( {0,{\pi \over 2}} \right)$$. If $$\mathop {\lim }\limits_{x \to 0 + } xy(x) = 1$$, then the value of $$y\left( {{\pi \over 4}} \right)$$ is :
3
Let A and B be independent events such that P(A) = p, P(B) = 2p. The largest value of p, for which P (exactly one of A, B occurs) = $${5 \over 9}$$, is :
4
Let $$\theta \in \left( {0,{\pi \over 2}} \right)$$. If the system of linear equations
$$(1 + {\cos ^2}\theta )x + {\sin ^2}\theta y + 4\sin 3\,\theta z = 0$$
$${\cos ^2}\theta x + (1 + {\sin ^2}\theta )y + 4\sin 3\,\theta z = 0$$
$${\cos ^2}\theta x + {\sin ^2}\theta y + (1 + 4\sin 3\,\theta )z = 0$$
has a non-trivial solution, then the value of $$\theta$$ is :
$$(1 + {\cos ^2}\theta )x + {\sin ^2}\theta y + 4\sin 3\,\theta z = 0$$
$${\cos ^2}\theta x + (1 + {\sin ^2}\theta )y + 4\sin 3\,\theta z = 0$$
$${\cos ^2}\theta x + {\sin ^2}\theta y + (1 + 4\sin 3\,\theta )z = 0$$
has a non-trivial solution, then the value of $$\theta$$ is :
5
Let $$f(x) = \cos \left( {2{{\tan }^{ - 1}}\sin \left( {{{\cot }^{ - 1}}\sqrt {{{1 - x} \over x}} } \right)} \right)$$, 0 < x < 1. Then :
6
Out of all the patients in a hospital 89% are found to be suffering from heart ailment and 98% are suffering from lungs infection. If K% of them are suffering from both ailments, then K can not belong to the set :
7
The equation $$\arg \left( {{{z - 1} \over {z + 1}}} \right) = {\pi \over 4}$$ represents a circle with :
8
If a line along a chord of the circle 4x2 + 4y2 + 120x + 675 = 0, passes through the point ($$-$$30, 0) and is tangent to the parabola y2 = 30x, then the length of this chord is :
9
The value of $$\int\limits_{{{ - 1} \over {\sqrt 2 }}}^{{1 \over {\sqrt 2 }}} {{{\left( {{{\left( {{{x + 1} \over {x - 1}}} \right)}^2} + {{\left( {{{x - 1} \over {x + 1}}} \right)}^2} - 2} \right)}^{{1 \over 2}}}dx} $$ is :
10
If $$A = \left( {\matrix{
{{1 \over {\sqrt 5 }}} & {{2 \over {\sqrt 5 }}} \cr
{{{ - 2} \over {\sqrt 5 }}} & {{1 \over {\sqrt 5 }}} \cr
} } \right)$$, $$B = \left( {\matrix{
1 & 0 \cr
i & 1 \cr
} } \right)$$, $$i = \sqrt { - 1} $$, and Q = ATBA, then the inverse of the matrix A Q2021 AT is equal to :
11
If the sum of an infinite GP a, ar, ar2, ar3, ....... is 15 and the sum of the squares of its each term is 150, then the sum of ar2, ar4, ar6, ....... is :
12
Let $$z = {{1 - i\sqrt 3 } \over 2}$$, $$i = \sqrt { - 1} $$. Then the value of $$21 + {\left( {z + {1 \over z}} \right)^3} + {\left( {{z^2} + {1 \over {{z^2}}}} \right)^3} + {\left( {{z^3} + {1 \over {{z^3}}}} \right)^3} + .... + {\left( {{z^{21}} + {1 \over {{z^{21}}}}} \right)^3}$$ is ______________.
13
The sum of all integral values of k (k $$\ne$$ 0) for which the equation $${2 \over {x - 1}} - {1 \over {x - 2}} = {2 \over k}$$ in x has no real roots, is ____________.
14
If $${}^1{P_1} + 2.{}^2{P_2} + 3.{}^3{P_3} + .... + 15.{}^{15}{P_{15}} = {}^q{P_r} - s,0 \le s \le 1$$, then $${}^{q + s}{C_{r - s}}$$ is equal to ______________.
15
A wire of length 36 m is cut into two pieces, one of the pieces is bent to form a square and the other is bent to form a circle. If the sum of the areas of the two figures is minimum, and the circumference of the circle is k (meter), then $$\left( {{4 \over \pi } + 1} \right)k$$ is equal to _____________.
16
The area of the region $$S = \{ (x,y):3{x^2} \le 4y \le 6x + 24\} $$ is ____________.
17
The locus of a point, which moves such that the sum of squares of its distances from the points (0, 0), (1, 0), (0, 1), (1, 1) is 18 units, is a circle of diameter d. Then d2 is equal to _____________.
18
If y = y(x) is an implicit function of x such that loge(x + y) = 4xy, then $${{{d^2}y} \over {d{x^2}}}$$ at x = 0 is equal to ___________.
19
The number of three-digit even numbers, formed by the digits 0, 1, 3, 4, 6, 7 if the repetition of digits is not allowed, is ______________.
20
Let a, b $$\in$$ R, b $$\in$$ 0, Define a function
$$f(x) = \left\{ {\matrix{ {a\sin {\pi \over 2}(x - 1),} & {for\,x \le 0} \cr {{{\tan 2x - \sin 2x} \over {b{x^3}}},} & {for\,x > 0} \cr } } \right.$$.
If f is continuous at x = 0, then 10 $$-$$ ab is equal to ________________.
$$f(x) = \left\{ {\matrix{ {a\sin {\pi \over 2}(x - 1),} & {for\,x \le 0} \cr {{{\tan 2x - \sin 2x} \over {b{x^3}}},} & {for\,x > 0} \cr } } \right.$$.
If f is continuous at x = 0, then 10 $$-$$ ab is equal to ________________.
Physics
1
The fractional change in the magnetic field intensity at a distance 'r' from centre on the axis of current carrying coil of radius 'a' to the magnetic field intensity at the centre of the same coil is : (Take r < a)
2
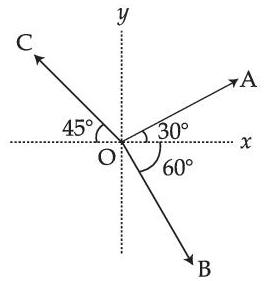
The magnitude of vectors $$\overrightarrow {OA} $$, $$\overrightarrow {OB} $$ and $$\overrightarrow {OC} $$ in the given figure are equal. The direction of $$\overrightarrow {OA} $$ + $$\overrightarrow {OB} $$ $$-$$ $$\overrightarrow {OC} $$ with x-axis will be :

3
Car B overtakes another car A at a relative speed of 40 ms$$-$$1. How fast will the image of car B appear to move in the mirror of focal length 10 cm fitted in car A, when the car B is 1.9 m away from the car A?
4
Inside a uniform spherical shell :
(1) the gravitational field is zero
(2) the gravitational potential is zero
(3) the gravitational field is same everywhere
(4) the gravitational potential is same everywhere
(5) all of the above
Choose the most appropriate answer from the options given below :
(1) the gravitational field is zero
(2) the gravitational potential is zero
(3) the gravitational field is same everywhere
(4) the gravitational potential is same everywhere
(5) all of the above
Choose the most appropriate answer from the options given below :
5
Two narrow bores of diameter 5.0 mm and 8.0 mm are joined together to form a U-shaped tube open at both ends. If this U-tube contains water, what is the difference in the level of two limbs of the tube. [Take surface tension of water T = 7.3 $$\times$$ 10$$-$$2 Nm$$-$$1, angle of contact = 0, g = 10 ms2 and density of water = 1.0 $$\times$$ 103 kg m$$-$$3]
6
An electric appliance supplies 6000 J/min heat to the system. If the system delivers a power of 90W. How long it would take to increase the internal energy by 2.5 $$\times$$ 103 J ?
7
An inductor coil stores 64 J of magnetic field energy and dissipates energy at the rate of 640 W when a current of 8A is passed through it. If this coil is joined across an ideal battery, find the time constant of the circuit in seconds :
8
A series LCR circuit driven by 300 V at a frequency of 50 Hz contains a resistance R = 3 k$$\Omega$$, an inductor of inductive reactance XL = 250 $$\pi$$$$\Omega$$ and an unknown capacitor. The value of capacitance to maximize the average power should be : (Take $$\pi$$2 = 10)
9
Identify the logic operation carried out by the given circuit :-


10
A particular hydrogen like ion emits radiation of frequency 2.92 $$\times$$ 1015 Hz when it makes transition from n = 3 to n = 1. The frequency in Hz of radiation emitted in transition from n = 2 to n = 1 will be :
11
In a photoelectric experiment ultraviolet light of wavelength 280 nm is used with lithium cathode having work function $$\phi$$ = 2.5 eV. If the wavelength of incident light is switched to 400 nm, find out the change in the stopping potential. (h = 6.63 $$\times$$ 10$$-$$34 Js, c = 3 $$\times$$ 108 ms$$-$$1)
12
In the given figure, the emf of the cell is 2.2 V and if internal resistance is 0.6$$\Omega$$. Calculate the power dissipated in the whole circuit :


13
A solid metal sphere of radius R having charge q is enclosed inside the concentric spherical shell of inner radius a and outer radius b as shown in the figure. The approximate variation electric field $$\overrightarrow E $$ as a function of distance r from centre O is given by


14
The rms speeds of the molecules of Hydrogen, Oxygen and Carbon dioxide at the same temperature are VH, VO and VC respectively then :
15
In a Screw Gauge, fifth division of the circular scale coincides with the reference line when the ratchet is closed. There are 50 divisions on the circular scale, and the main scale moves by 0.5 mm on a complete rotation. For a particular observation the reading on the main scale is 5 mm and the 20th division of the circular scale coincides with reference line. Calculate the true reading.
16
What equal length of an iron wire and a copper-nickel alloy wire, each of 2 mm diameter connected parallel to give an equivalent resistance of 3$$\Omega$$ ?
(Given resistivities of iron and copper-nickel alloy wire are 12 $$\mu$$$$\Omega$$ and 51 $$\mu$$$$\Omega$$ cm respectively)
(Given resistivities of iron and copper-nickel alloy wire are 12 $$\mu$$$$\Omega$$ and 51 $$\mu$$$$\Omega$$ cm respectively)
17
The initial mass of a rocket is 1000 kg. Calculate at what rate the fuel should be burnt so that the rocket is given an acceleration of 20 ms-2. The gases come out at a relative speed of 500 ms$$-$$1 with respect to the rocket : [Use g = 10 m/s2]
18
If E, L, M and G denote the quantities as energy, angular momentum, mass and constant of gravitation respectively, then the dimensions of P in the formula P = EL2M$$-$$5G$$-$$2 are :
19
The material filled between the plates of a parallel plate capacitor has resistivity 200 $$\Omega$$m. The value of capacitance of the capacitor is 2 pF. If a potential difference of 40 V is applied across the plates of the capacitor, then the value of leakage current flowing out of the capacitor is : (given the value of relative permittivity of material is 50)
20
Statement I : By doping silicon semiconductor with pentavalent material, the electrons density increases.
Statement II : The n-type semiconductor has net negative charge.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement II : The n-type semiconductor has net negative charge.
In the light of the above statements, choose the most appropriate answer from the options given below :
21
A uniform chain of length 3 meter and mass 3 kg overhangs a smooth table with 2 meter lying on the table. If k is the kinetic energy of the chain in joule as it completely slips off the table, then the value of k is ................. . (Take g = 10 m/s2)
22
The electric field in a plane electromagnetic wave is given by
$$\overrightarrow E = 200\cos \left[ {\left( {{{0.5 \times {{10}^3}} \over m}} \right)x - \left( {1.5 \times {{10}^{11}}{{rad} \over s} \times t} \right)} \right]{V \over m}\widehat j$$. If this wave falls normally on a perfectly reflecting surface having an area of 100 cm2. If the radiation pressure exerted by the E.M. wave on the surface during a 10 minute exposure is $${x \over {{{10}^9}}}{N \over {{m^2}}}$$. Find the value of x .
$$\overrightarrow E = 200\cos \left[ {\left( {{{0.5 \times {{10}^3}} \over m}} \right)x - \left( {1.5 \times {{10}^{11}}{{rad} \over s} \times t} \right)} \right]{V \over m}\widehat j$$. If this wave falls normally on a perfectly reflecting surface having an area of 100 cm2. If the radiation pressure exerted by the E.M. wave on the surface during a 10 minute exposure is $${x \over {{{10}^9}}}{N \over {{m^2}}}$$. Find the value of x .
23
Two spherical balls having equal masses with radius of 5 cm each are thrown upwards along the same vertical direction at an interval of 3s with the same initial velocity of 35 m/s, then these balls collide at a height of ............... m. (Take g = 10 m/s2)
24
A soap bubble of radius 3 cm is formed inside the another soap bubble of radius 6 cm. The radius of an equivalent soap bubble which has the same excess pressure as inside the smaller bubble with respect to the atmospheric pressure is ................ cm.
25
Two short magnetic dipoles m1 and m2 each having magnetic moment of 1 Am2 are placed at point O and P respectively. The distance between OP is 1 meter. The torque experienced by the magnetic dipole m2 due to the presence of m1 is ........... $$\times$$ 10$$-$$7 Nm.
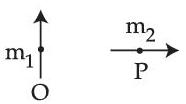
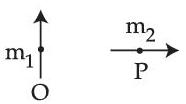
26
Two travelling waves produces a standing wave represented by equation,
y = 1.0 mm cos(1.57 cm$$-$$1) x sin(78.5 s$$-$$1)t.
The node closest to the origin in the region x > 0 will be at x = .............. cm.
y = 1.0 mm cos(1.57 cm$$-$$1) x sin(78.5 s$$-$$1)t.
The node closest to the origin in the region x > 0 will be at x = .............. cm.
27
White light is passed through a double slit and interference is observed on a screen 1.5 m away. The separation between the slits is 0.3 mm. The first violet and red fringes are formed 2.0 mm and 3.5 mm away from the central white fringes. the difference in wavelengths of red and violet light is ................ nm.
28
Consider a badminton racket with length scales as shown in the figure.
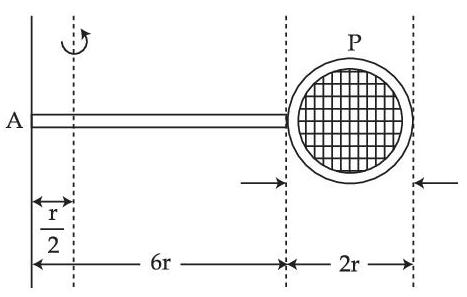
If the mass of the linear and circular portions of the badminton racket are same (M) and the mass of the threads are negligible, the moment of inertia of the racket about an axis perpendicular to the handle and in the plane of the ring at, $${r \over 2}$$ distance from the end A of the handle will be ................ Mr2.

If the mass of the linear and circular portions of the badminton racket are same (M) and the mass of the threads are negligible, the moment of inertia of the racket about an axis perpendicular to the handle and in the plane of the ring at, $${r \over 2}$$ distance from the end A of the handle will be ................ Mr2.
1
JEE Main 2021 (Online) 26th August Morning Shift
MCQ (Single Correct Answer)
+4
-1
The correct options for the products A and B of the following reactions are :


2
JEE Main 2021 (Online) 26th August Morning Shift
MCQ (Single Correct Answer)
+4
-1
Given below are two statements.
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with NaOH phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with NaOH phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below :
3
JEE Main 2021 (Online) 26th August Morning Shift
MCQ (Single Correct Answer)
+4
-1
Among the following compounds I-IV, which one forms a yellow precipitate on reacting sequentially with (i) NaOH (ii) dil. HNO3 (iii) AgNO3?


4
JEE Main 2021 (Online) 26th August Morning Shift
MCQ (Single Correct Answer)
+4
-1
Given below are two statements.
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below :
Subject
Chemistry
22
Mathematics
20
Physics
28
More Papers of JEE Main
2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002


