2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002JEE Main 2019 (Online) 9th April Evening Slot
Paper was held on Tue, Apr 9, 2019 9:30 AM
Chemistry
1
Hinsberg's reagent is :
2
Molal depression constant for a solvent is
4.0 kg mol–1. The depression in the freezing
point of the solvent for 0.03 mol kg–1 solution
of K2SO4 is :
(Assume complete dissociation of the electrolyte)
(Assume complete dissociation of the electrolyte)
3
The maximum number of possible oxidation
states of actinoides are shown by :
4
The major product of the following reaction is:


5
Which of the following potential energy (PE)
diagrams represents the SN1 reaction?
6
In the following reaction


7
Consider the given plot of enthalpy of the
following reaction between A and B.
A+ B $$ \to $$ C + D
Identify the incorrect statement.
A+ B $$ \to $$ C + D
Identify the incorrect statement.

8
A solution of Ni(NO3)2 is electrolysed between
platinum electrodes using 0.1 Faraday
electricity. How many mole of Ni will be
deposited at the cathode?
9
What would be the molality of 20% (mass/
mass) aqueous solution of KI?
(molar mass of KI = 166 g mol–1)
(molar mass of KI = 166 g mol–1)
10
The peptide that gives positive ceric
ammonium nitrate and carbylamine tests is :
11
Among the following species, the diamagnetic
molecule is
12
The correct statements among I to III regarding
group 13 element oxides are,
(I) Boron trioxide is acidic.
(II) Oxides of aluminium and gallium are amphoteric.
(III) Oxides of indium and thalliumare basic.
(I) Boron trioxide is acidic.
(II) Oxides of aluminium and gallium are amphoteric.
(III) Oxides of indium and thalliumare basic.
13
The maximum possible denticities of a ligand
given below towards a common transition and
inner-transition metal ion, respectively, are :


14
Which one of the following about an electron
occupying the 1s orbital in a hydrogen atom is
incorrect ?
(The Bohr radius is represented by a0)
(The Bohr radius is represented by a0)
15
The major products A and B for the following
reactions are, respectively:


16
During compression of a spring the work done
is 10kJ and 2kJ escaped to the surroundings as
heat. The change in internal energy, $$\Delta $$U(inkJ)
is :
17
Increasing order of reactivity of the following
compounds for SN1 substitution is:


18
HF has highest boiling point among hydrogen
halides, because it has :
19
In an acid-base titration, 0.1 M HCl solution
was added to the NaOH solution of unknown
strength. Which of the following correctly
shows the change of pH of the titraction
mixture in this experiment?


20
p-Hydroxybenzophenone upon reaction with
bromine in carbon tetrachloride gives:
21
The correct statements among I to III are :
(I) Valence bond theory cannot explain the color exhibited by transition metal complexes.
(II) Valence bond theory can predict quantitatively the magnetic properties of transtition metal complexes.
(III) Valence bond theory cannot distinguish ligands as weak and strong field ones.
(I) Valence bond theory cannot explain the color exhibited by transition metal complexes.
(II) Valence bond theory can predict quantitatively the magnetic properties of transtition metal complexes.
(III) Valence bond theory cannot distinguish ligands as weak and strong field ones.
Mathematics
1
The value of the integral $$\int\limits_0^1 {x{{\cot }^{ - 1}}(1 - {x^2} + {x^4})dx} $$ is :-
2
If the system of equations 2x + 3y – z = 0, x + ky
– 2z = 0 and 2x – y + z = 0 has a non-trival solution
(x, y, z), then $${x \over y} + {y \over z} + {z \over x} + k$$
is equal to :-
3
The value of sin 10º sin30º sin50º sin70º is :-
4
If $$\cos x{{dy} \over {dx}} - y\sin x = 6x$$, (0 < x < $${\pi \over 2}$$)
and $$y\left( {{\pi \over 3}} \right)$$ = 0 then $$y\left( {{\pi \over 6}} \right)$$ is equal to :-
and $$y\left( {{\pi \over 3}} \right)$$ = 0 then $$y\left( {{\pi \over 6}} \right)$$ is equal to :-
5
Let z $$ \in $$ C be such that |z| < 1.
If $$\omega = {{5 + 3z} \over {5(1 - z)}}$$z, then :
If $$\omega = {{5 + 3z} \over {5(1 - z)}}$$z, then :
6
A rectangle is inscribed in a circle with a diameter
lying along the line 3y = x + 7. If the two adjacent
vertices of the rectangle are (–8, 5) and (6, 5), then
the area of the rectangle (in sq. units) is :
7
If a unit vector $$\overrightarrow a $$ makes angles $$\pi $$/3 with $$\widehat i$$ , $$\pi $$/ 4
with $$\widehat j$$ and $$\theta $$$$ \in $$(0, $$\pi $$) with $$\widehat k$$, then a value of $$\theta $$
is :-
8
The mean and the median of the following ten
numbers in increasing order 10, 22, 26, 29, 34, x,
42, 67, 70, y are 42 and 35 respectively, then $${y \over x}$$ is equal to
9
The area (in sq. units) of the region
A = {(x, y) : $${{y{}^2} \over 2}$$ $$ \le $$ x $$ \le $$ y + 4} is :-
A = {(x, y) : $${{y{}^2} \over 2}$$ $$ \le $$ x $$ \le $$ y + 4} is :-
10
The domain of the definition of the function
$$f(x) = {1 \over {4 - {x^2}}} + {\log _{10}}({x^3} - x)$$ is
$$f(x) = {1 \over {4 - {x^2}}} + {\log _{10}}({x^3} - x)$$ is
11
Two newspapers A and B are published in a city.
It is known that 25% of the city populations reads
A and 20% reads B while 8% reads both A and
B. Further, 30% of those who read A but not B
look into advertisements and 40% of those who
read B but not A also look into advertisements,
while 50% of those who read both A and B look
into advertisements. Then the percentage of the
population who look into advertisement is :-
12
If m is chosen in the quadratic equation
(m2 + 1) x2 – 3x + (m2 + 1)2 = 0
such that the sum of its roots is greatest, then the absolute difference of the cubes of its roots is :-
(m2 + 1) x2 – 3x + (m2 + 1)2 = 0
such that the sum of its roots is greatest, then the absolute difference of the cubes of its roots is :-
13
$$\int {{e^{\sec x}}}$$ $$(\sec x\tan xf(x) + \sec x\tan x + se{x^2}x)dx$$
= esecxf(x) + C then a possible choice of f(x) is :-
= esecxf(x) + C then a possible choice of f(x) is :-
14
If the function $$f(x) = \left\{ {\matrix{
{a|\pi - x| + 1,x \le 5} \cr
{b|x - \pi | + 3,x > 5} \cr
} } \right.$$
is continuous at x = 5, then the value of a – b is :-
is continuous at x = 5, then the value of a – b is :-
15
If $$f(x) = [x] - \left[ {{x \over 4}} \right]$$ ,x $$ \in $$
4
, where [x] denotes the
greatest integer function, then
16
The total number of matrices
$$A = \left( {\matrix{ 0 & {2y} & 1 \cr {2x} & y & { - 1} \cr {2x} & { - y} & 1 \cr } } \right)$$
(x, y $$ \in $$ R,x $$ \ne $$ y) for which ATA = 3I3 is :-
$$A = \left( {\matrix{ 0 & {2y} & 1 \cr {2x} & y & { - 1} \cr {2x} & { - y} & 1 \cr } } \right)$$
(x, y $$ \in $$ R,x $$ \ne $$ y) for which ATA = 3I3 is :-
17
The vertices B and C of a $$\Delta $$ABC lie on the line,
$${{x + 2} \over 3} = {{y - 1} \over 0} = {z \over 4}$$ such that BC = 5 units.
Then the area (in sq. units) of this triangle, given that the point A(1, –1, 2), is :
$${{x + 2} \over 3} = {{y - 1} \over 0} = {z \over 4}$$ such that BC = 5 units.
Then the area (in sq. units) of this triangle, given that the point A(1, –1, 2), is :
18
A water tank has the shape of an inverted right
circular cone, whose semi-vertical angle is
$${\tan ^{ - 1}}\left( {{1 \over 2}} \right)$$. Water is poured into it at a constant
rate of 5 cubic meter per minute. The the rate
(in m/min.), at which the level of water is rising
at the instant when the depth of water in the tank
is 10m; is :-
19
If the two lines x + (a – 1) y = 1 and
2x + a2y = 1 (a$$ \in $$R – {0, 1}) are perpendicular, then
the distance of their point of intersection from the
origin is :
20
If f : R $$ \to $$ R is a differentiable function and
f(2) = 6,
then $$\mathop {\lim }\limits_{x \to 2} {{\int\limits_6^{f\left( x \right)} {2tdt} } \over {\left( {x - 2} \right)}}$$ is :-
then $$\mathop {\lim }\limits_{x \to 2} {{\int\limits_6^{f\left( x \right)} {2tdt} } \over {\left( {x - 2} \right)}}$$ is :-
21
If the sum and product of the first three term in
an A.P. are 33 and 1155, respectively, then a value
of its 11th term is :-
Physics
1
A thin smooth rod of length L and mass M is
rotating freely with angular speed $$\omega $$0 about an
axis perpendicular to the rod and passing
through its center. Two beads of mass m and
negligible size are at the center of the rod
initially. The beads are free to slide along the
rod. The angular speed of the system , when
the beads reach the opposite ends of the rod,
will be :-
2
A string 2.0 m long and fixed at its ends is
driven by a 240 Hz vibrator. The string vibrates
in its third harmonic mode. The speed of the
wave and its fundamental frequency is :-
3
In a conductor, if the number of conduction
electrons per unit volume is 8.5 × 1028 m–3 and
mean free time is 25ƒs (femto second), it's
approximate resistivity is :-
(me = 9.1 × 10–31 kg)
(me = 9.1 × 10–31 kg)
4
The parallel combination of two air filled
parallel plate capacitors of capacitance C and
nC is connected to a battery of voltage, V. When
the capacitors are fully charged, the battery is
removed and after that a dielectric material of
dielectric constant K is placed between the two
plates of the first capacitor. The new potential
difference of the combined system is :-
5
Two materials having coefficients of thermal
conductivity '3K' and 'K' and thickness 'd' and
'3d', respectively, are joined to form a slab as
shown in the figure. The temperatures of the
outer surfaces are '$$\theta $$2' and '$$\theta $$1' respectively,
($$\theta $$2 > $$\theta $$1). The temperature at the interface is :-
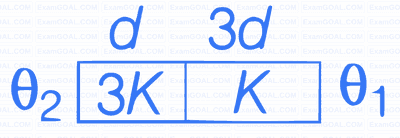
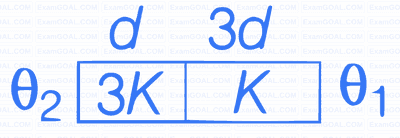
6
A He+ ion is in its first excited state. Its
ionization energy is :-
7
A wedge of mass M = 4m lies on a frictionless
plane. A particle of mass m approaches the
wedge with speed v. There is no friction
between the particle and the plane or between
the particle and the wedge. The maximum
height climbed by the particle on the wedge is
given by :-
8
A particle of mass 'm' is moving with speed '2v'
and collides with a mass '2m' moving with
speed 'v' in the same direction. After collision,
the first mass is stopped completely while the
second one splits into two particles each of
mass 'm', which move at angle 45° with respect
to the origianl direction.
The speed of each of the moving particle will
be :-
9
A wooden block floating in a bucket of water
has 4/5 of its volume submerged. When certain
amount of an oil is poured into the bucket, it
is found that the block is just under the oil
surface with half of its volume under water and
half in oil. The density of oil relative to that of
water is :-
10
A test particle is moving in a circular orbit in
the gravitational field produced by a mass
density $$\rho (r) = {K \over {{r^2}}}$$ . Identify the correct relation
between the radius R of the particle's orbit and
its period T
11
The position vector of a particle changes with
time according to the relation
$$\overrightarrow r (t) = 15{t^2}\widehat i + (4 - 20{t^2})\widehat j$$
What is the magnitude of the acceleration at t = 1 ?
What is the magnitude of the acceleration at t = 1 ?
12
The specific heats, CP and CV of a gas of
diatomic molecules, A, are given (in units of
J mol–1 K–1) by 29 and 22, respectively.
Another gas of diatomic molecules, B, has the
corresponding values 30 and 21. If they are
treated as ideal gases, then :-
13
Four point charges –q, +q, +q and –q are placed
on y-axis at y = –2d, y = –d, y = +d and
y = +2d, respectively. The magnitude of the
electric field E at a point on the x-axis at
x = D, with D >> d, will behave as :-
14
A moving coil galvanometer has a coil with
175 turns and area 1 cm2. It uses a torsion band
of torsion constant 10–6 N-m/rad. The coil is
placed in a maganetic field B parallel to its
plane. The coil deflects by 1° for a current of
1 mA. The value of B (in Tesla) is
approximately :-
15
50 W/m2 energy density of sunlight is normally
incident on the surface of a solar panel. Some
part of incident energy (25%) is reflected from
the surface and the rest is absorbed. The force
exerted on 1m2 surface area will be close to
(c = 3 × 108 m/s) :-
16
The area of a square is 5.29 cm2. The area of
7 such squares taking into account the
significant figures is :-
17
The logic gate equivalent to the given logic
circuit is :-


18
Moment of inertia of a body about a given axis
is 1.5 kg m2. Initially the body is at rest. In order
to produce a rotational kinetic energy of
1200 J, the angular accleration of 20 rad/s2
must be applied about the axis for a
duration of :-
19
A particle 'P' is formed due to a completely
inelastic collision of particles 'x' and 'y' having
de-Broglie wavelengths '$$\lambda $$x' and '$$\lambda $$y'
respectively. If x and y were moving in opposite
directions, then the de-Broglie wavelength of
'P' is :-
20
A convex lens of focal length 20 cm produces
images of the same magnification 2 when an
object is kept at two distances x1 and x2
(x1 > x2) from the lens. The ratio of x1 and x2
is :-
21
Diameter of the objective lens of a telescope is
250 cm. For light of wavelength 600nm.
coming from a distant object, the limit of
resolution of the telescope is close to :-
22
A massless spring (k = 800 N/m), attached with
a mass (500 g) is completely immersed in 1 kg
of water. The spring is stretched by 2 cm and
released so that it starts vibrating. What would
be the order of magnitude of the change in the
temperature of water when the vibrations stop
completely ? (Assume that the water container
and spring receive negligible heat and specific
heat of mass = 400 J/kg K, specific heat of
water = 4184 J/kg K)
23
A very long solenoid of radius R is carrying
current I(t) = kte–at(k > 0), as a function of time
(t $$ \ge $$ 0). counter clockwise current is taken to be
positive. A circular conducting coil of radius
2R is placed in the equatorial plane of the
solenoid and concentric with the solenoid. The
current induced in the outer coil is correctly
depicted, as a function of time, by :-
24
The position of a particle as a function of time
t, is given by
x(t) = at + bt2 – ct3
where a, b and c are constants. When the particle attains zero acceleration, then its velocity will be :
x(t) = at + bt2 – ct3
where a, b and c are constants. When the particle attains zero acceleration, then its velocity will be :
25
A thin convex lens L (refractive index = 1.5)
is placed on a plane mirror M. When a pin is
placed at A, such that OA = 18 cm, its real
inverted image is formed at A itself, as shown
in figure. When a liquid of refractive index μ1
is put between the lens and the mirror, The pin
has to be moved to A', such that OA' = 27 cm,
to get its inverted real image at A' itself. The
value of μ1 will be :-


26
The resistance of a galvanometer is 50 ohm and
the maximum current which can be passed
through it is 0.002 A. What resistance must be
connected to it in order to convert it into an
ammeter of range 0 – 0.5 A ?
27
A metal wire of resistance 3 $$\Omega $$ is elongated to
make a uniform wire of double its previous
length. This new wire is now bent and the ends
joined to make a circle. If two points on this
circle make an angle 60° at the centre, the
equivalent resistance between these two points
will be :-
28
Two coils 'P' and 'Q' are separated by some
distance. When a current of 3 A flows through
coil 'P', a magnetic flux of 10–3 Wb passes
through 'Q'. No current is passed through 'Q'.
When no current passes through 'P' and a
current of 2 A passes through 'Q', the flux
through 'P' is :-