2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002JEE Main 2019 (Online) 10th January Evening Slot
Paper was held on Thu, Jan 10, 2019 9:30 AM
Chemistry
1
The major product of the following reaction


2
Which is the most suitable reagent for the following transformation ?


3
The major product obtained in the following reaction is :


4
What will be the major product in the following mononitration reaction ?


5
The major product of the following reaction is :


6
For an elementary chemical reaction,

the expression for $${{d\left[ A \right]} \over {dt}}$$ is

the expression for $${{d\left[ A \right]} \over {dt}}$$ is
7
What is the IUPAC name of the following compound ?


8
An aromatic compound 'A' having molecular formula C7H6O2 on treating with aqueous ammonia and heating forms compound 'B'. The compound 'B' on reaction with molecular bromine and potassium hydroxide provides compound 'C' having molecular formula C6H7N.. The structure of 'A' is
9
A reaction of cobalt (III) chloride and ethylenediamine in a 1 : 2 mole ratio generates two isomeric products A (violet coloured) and B (green coloured). A can show optical activity, but B is optically inactive. What
type of isomers does A and B represcent?
10
The pair that contains two P – H bonds in each of the oxoacids is
11
In the cell
Pt$$\left| {\left( s \right)} \right|$$H2(g, 1 bar)$$\left| {HCl\left( {aq} \right)} \right|$$AgCl$$\left| {\left( s \right)} \right|$$Ag(s)|Pt(s)
the cell potential is 0.92 V when a 10–6 molal HCl solution is used. The standard electrode potential of (AgCl/ AgCl– ) electrode is :
$$\left\{ {} \right.$$Given, $${{2.303RT} \over F} = 0.06V$$ at $$\left. {298} \right\}$$
Pt$$\left| {\left( s \right)} \right|$$H2(g, 1 bar)$$\left| {HCl\left( {aq} \right)} \right|$$AgCl$$\left| {\left( s \right)} \right|$$Ag(s)|Pt(s)
the cell potential is 0.92 V when a 10–6 molal HCl solution is used. The standard electrode potential of (AgCl/ AgCl– ) electrode is :
$$\left\{ {} \right.$$Given, $${{2.303RT} \over F} = 0.06V$$ at $$\left. {298} \right\}$$
12
5.1 g NH4SH is introduced in 3.0 L evacuated flask at 327ºC. 30% of the solid NH4SH decomposed to NH3 and H2S as gases . The Kp of the reaction at 327oC is (R = 0.082 L atm mol–1 K–1, Molar mass of S = 32 g mol–1 molar mass of N = 14 g mol–1)
13
The correct match between item 'I' and item 'II' is :
| Item 'I' (compound) | Item 'II' (reagent) |
||
|---|---|---|---|
| (A) | Lysine | (P) | 1-naphthol |
| (B) | Furfural | (Q) | ninhydrin |
| (C) | Benzylalcohol | (R) | KMnO4 |
| (D) | Styrene | (S) | Ceric ammonium nitrate |
14
An ideal gas undergoes isothermal compression from 5m3 to 1 m3 against a constant external pressure of 4 Nm–2. Heat released in this process is used to increase the temperature of 1 mole of Al. If molar heat capacity of Al is 24 J mol–1 K–1, the temperature of Al increases by :
15
Elevation in the boiling point for 1 molar solution of glucose is 2 K. The depression in the freezing point for 2 molal solution of glucose in the same solvent is 2 K. The relation between Kb and Kf is
16
The amount of sugar (C12H22O11) required to prepare 2L of its 0.1 M aqueous solution is :
17
The process with negative entropy change is :
18
The difference in the number of unpaired electrons of a metal ion in its high spin and low-spin octahedral complexes is two. The metal ion is :
19
The 71st electron of an element X with an atomic number of 71 enters into the orbital :
20
The ground state energy of hydrogen atom is – 13.6 eV. The energy of second excited state of He+ ion in eV is :
21
Which of the following tests cannot be used for identifying amino acids?
22
In the reaction of oxalate with permanganate in acidic medium, the number of electrons involved in producing one molecule of CO2 is :
Mathematics
1
If $$\int\limits_0^x \, $$f(t) dt = x2 + $$\int\limits_x^1 \, $$ t2f(t) dt then f '$$\left( {{1 \over 2}} \right)$$ is -
2
Let f be a differentiable function such that f '(x) = 7 - $${3 \over 4}{{f\left( x \right)} \over x},$$ (x > 0) and f(1) $$ \ne $$ 4. Then $$\mathop {\lim }\limits_{x \to 0'} \,$$ xf$$\left( {{1 \over x}} \right)$$ :
3
Let f : ($$-$$1, 1) $$ \to $$ R be a function defined by f(x) = max $$\left\{ { - \left| x \right|, - \sqrt {1 - {x^2}} } \right\}.$$ If K be the set of all points at which f is not differentiable, then K has exactly -
4
The number of values of $$\theta $$ $$ \in $$ (0, $$\pi $$) for which the system of linear equations
x + 3y + 7z = 0
$$-$$ x + 4y + 7z = 0
(sin3$$\theta $$)x + (cos2$$\theta $$)y + 2z = 0.
has a non-trival solution, is -
x + 3y + 7z = 0
$$-$$ x + 4y + 7z = 0
(sin3$$\theta $$)x + (cos2$$\theta $$)y + 2z = 0.
has a non-trival solution, is -
5
The curve amongst the family of curves represented by the differential equation, (x2 – y2)dx + 2xy dy = 0 which passes through (1, 1) is :
6
Let $$z = {\left( {{{\sqrt 3 } \over 2} + {i \over 2}} \right)^5} + {\left( {{{\sqrt 3 } \over 2} - {i \over 2}} \right)^5}.$$ If R(z) and 1(z) respectively denote the real and imaginary parts of z, then :
7
The positive value of $$\lambda $$ for which the co-efficient of x2
in the expression x2 $${\left( {\sqrt x + {\lambda \over {{x^2}}}} \right)^{10}}$$ is 720, is -
8
The value of $$\lambda $$ such that sum of the squares of the roots of the quadratic equation, x2 + (3 – $$\lambda $$)x + 2 = $$\lambda $$ has the least value is -
9
Let A = $$\left[ {\matrix{
2 & b & 1 \cr
b & {{b^2} + 1} & b \cr
1 & b & 2 \cr
} } \right]$$ where b > 0.
Then the minimum value of $${{\det \left( A \right)} \over b}$$ is -
Then the minimum value of $${{\det \left( A \right)} \over b}$$ is -
10
If $$\overrightarrow \alpha $$ = $$\left( {\lambda - 2} \right)\overrightarrow a + \overrightarrow b $$ and $$\overrightarrow \beta = \left( {4\lambda - 2} \right)\overrightarrow a + 3\overrightarrow b $$ be two given vectors $$\overrightarrow a $$ and $$\overrightarrow b $$ are non-collinear. The value of $$\lambda $$ for which vectors $$\overrightarrow \alpha $$ and $$\overrightarrow \beta $$ are collinear, is -
11
Let S = $$\left\{ {\left( {x,y} \right) \in {R^2}:{{{y^2}} \over {1 + r}} - {{{x^2}} \over {1 - r}}} \right\};r \ne \pm 1.$$ Then S represents :
12
The value of $$\int\limits_{ - \pi /2}^{\pi /2} {{{dx} \over {\left[ x \right] + \left[ {\sin x} \right] + 4}}} ,$$ where [t] denotes the greatest integer less than or equal to t, is
13
Two vertices of a triangle are (0, 2) and (4, 3). If its orthocenter is at the origin, then its third vertex lies in which quadrant :
14
Let a1, a2, a3, ..... a10 be in G.P. with ai > 0 for i = 1, 2, ….., 10 and S be the set of pairs (r, k), r, k $$ \in $$ N (the set of natural numbers) for which
$$\left| {\matrix{ {{{\log }_e}\,{a_1}^r{a_2}^k} & {{{\log }_e}\,{a_2}^r{a_3}^k} & {{{\log }_e}\,{a_3}^r{a_4}^k} \cr {{{\log }_e}\,{a_4}^r{a_5}^k} & {{{\log }_e}\,{a_5}^r{a_6}^k} & {{{\log }_e}\,{a_6}^r{a_7}^k} \cr {{{\log }_e}\,{a_7}^r{a_8}^k} & {{{\log }_e}\,{a_8}^r{a_9}^k} & {{{\log }_e}\,{a_9}^r{a_{10}}^k} \cr } } \right|$$ $$=$$ 0.
Then the number of elements in S, is -
$$\left| {\matrix{ {{{\log }_e}\,{a_1}^r{a_2}^k} & {{{\log }_e}\,{a_2}^r{a_3}^k} & {{{\log }_e}\,{a_3}^r{a_4}^k} \cr {{{\log }_e}\,{a_4}^r{a_5}^k} & {{{\log }_e}\,{a_5}^r{a_6}^k} & {{{\log }_e}\,{a_6}^r{a_7}^k} \cr {{{\log }_e}\,{a_7}^r{a_8}^k} & {{{\log }_e}\,{a_8}^r{a_9}^k} & {{{\log }_e}\,{a_9}^r{a_{10}}^k} \cr } } \right|$$ $$=$$ 0.
Then the number of elements in S, is -
15
If $$\sum\limits_{r = 0}^{25} {\left\{ {{}^{50}{C_r}.{}^{50 - r}{C_{25 - r}}} \right\} = K\left( {^{50}{C_{25}}} \right)} ,\,\,$$ then K is equal to :
16
The value of $$\cos {\pi \over {{2^2}}}.\cos {\pi \over {{2^3}}}\,.....\cos {\pi \over {{2^{10}}}}.\sin {\pi \over {{2^{10}}}}$$ is -
17
If mean and standard deviation of 5 observations x1, x2, x3, x4, x5 are 10 and 3, respectively, then the variance of 6 observations x1, x2, ….., x5 and –50 is equal to
18
The value of $$\cot \left( {\sum\limits_{n = 1}^{19} {{{\cot }^{ - 1}}} \left( {1 + \sum\limits_{p = 1}^n {2p} } \right)} \right)$$ is :
19
Two sides of a parallelogram are along the lines, x + y = 3 & x – y + 3 = 0. If its diagonals intersect at (2, 4), then one of its vertex is :
20
A helicopter is flying along the curve given by y – x3/2 = 7, (x $$ \ge $$ 0). A soldier positioned at the point $$\left( {{1 \over 2},7} \right)$$ wants to shoot down the helicopter when it is nearest to him. Then this nearest distance is -
21
If $$\int \, $$x5.e$$-$$4x3 dx = $${1 \over {48}}$$e$$-$$4x3 f(x) + C, where C is a constant of inegration, then f(x) is equal to -
22
If the area of an equilateral triangle inscribed in the circle x2 + y2
+ 10x + 12y + c = 0 is $$27\sqrt 3 $$ sq units then c is equal to :
23
The length of the chord of the parabola x2 $$=$$ 4y having equation x – $$\sqrt 2 y + 4\sqrt 2 = 0$$ is -
24
Let N be the set of natural numbers and two functions f and g be defined as f, g : N $$ \to $$ N such that
f(n) = $$\left\{ {\matrix{ {{{n + 1} \over 2};} & {if\,\,n\,\,is\,\,odd} \cr {{n \over 2};} & {if\,\,n\,\,is\,\,even} \cr } \,\,} \right.$$;
and g(n) = n $$-$$($$-$$ 1)n.
Then fog is -
f(n) = $$\left\{ {\matrix{ {{{n + 1} \over 2};} & {if\,\,n\,\,is\,\,odd} \cr {{n \over 2};} & {if\,\,n\,\,is\,\,even} \cr } \,\,} \right.$$;
and g(n) = n $$-$$($$-$$ 1)n.
Then fog is -
Physics
1
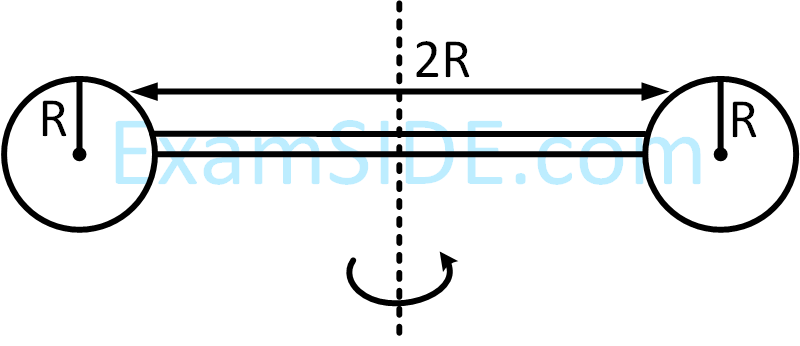
Two identical spherical balls of mass M and radius R each are stuck on two ends of a rod of length 2R and mass M (see figure). The moment of inertia of the system about the axis passing perpendicularly through the centre of the rod is :

2
The actual value of resistance R, shown in the figure is 30$$\Omega $$. This is measuered in an experiment as shown
using the standard formula R = $${V \over {\rm I}}$$, where V and I are the readings of the voltmeter and ammeter, respectively. If the measured value of R is 5% less, then the internal resistance of the voltmeter is -


3
The Wheatstone bridge shown in figure, here, gets balanced when the carbon resistor used as R1 has the colour code (Orange, Red, Brown). The resistors R2 and R4 are 80$$\Omega $$ and 40$$\Omega $$, respectively. Assuming that the colour code for the carbon resistors gives their accurate values, the colour code for the carbon resistor, used as R3, would be -


4
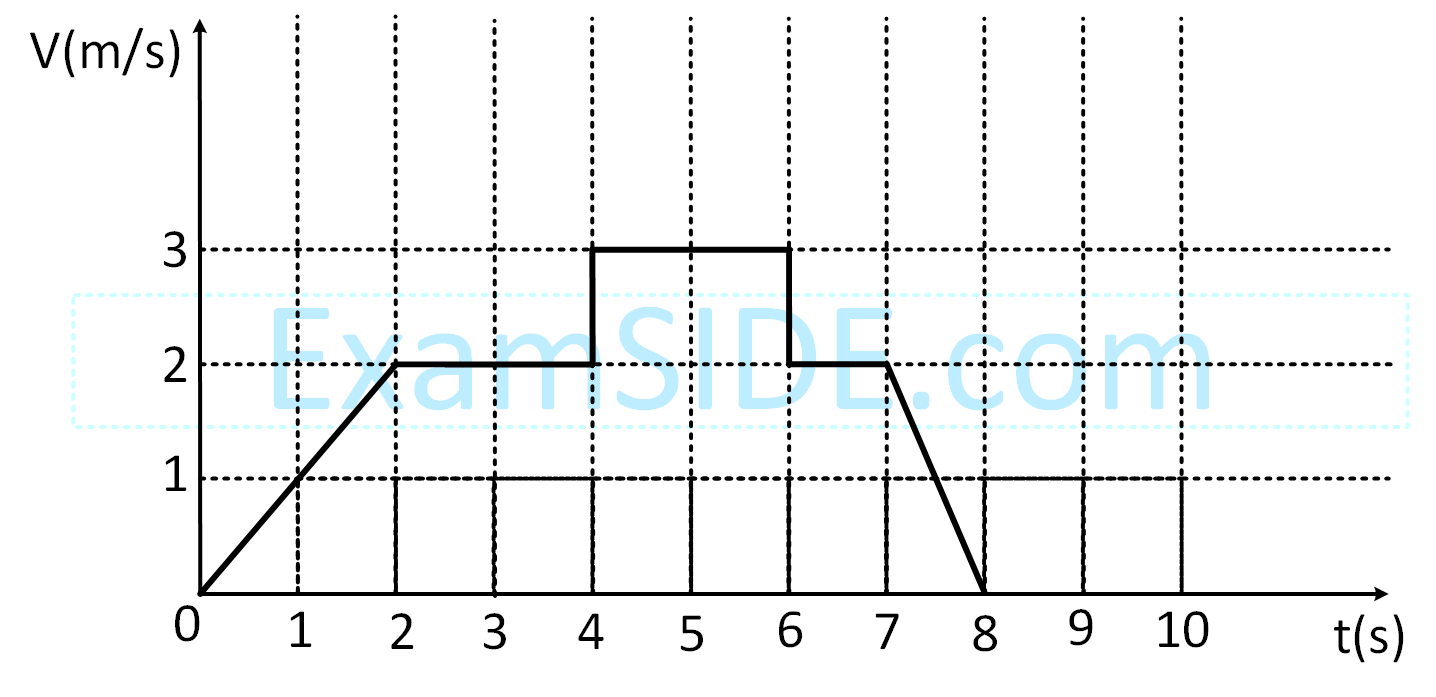
A particle starts from the origin at time t = 0 and moves along the positive x-axis. The graph of velocity with respect to time is shown in figure. What is the position of the particle at time t = 5s ?

5
Charges –q and +q located at A and B, respectively, constitude an electric dipole. Distance AB = 2a, O is the mid point of the dipole and OP is perpendicular to AB. A charge Q is placed at P where OP = y and y >> 2a. The charge Q experiences an electrostatic force F. If Q is now moved along the equatorial line to P' such that OP' = $$\left( {{y \over 3}} \right)$$, the force on Q will be close to - $$\left( {{y \over 3} > > 2a} \right)$$


6
Consider a Young’s double slit experiment as shown in figure. What should be the slit separation d in terms of wavelength $$\lambda $$ such that the first minima occurs directly in front of the slit (S1) ?


7
For the circuit shown below, the current through the Zener diode is -


8
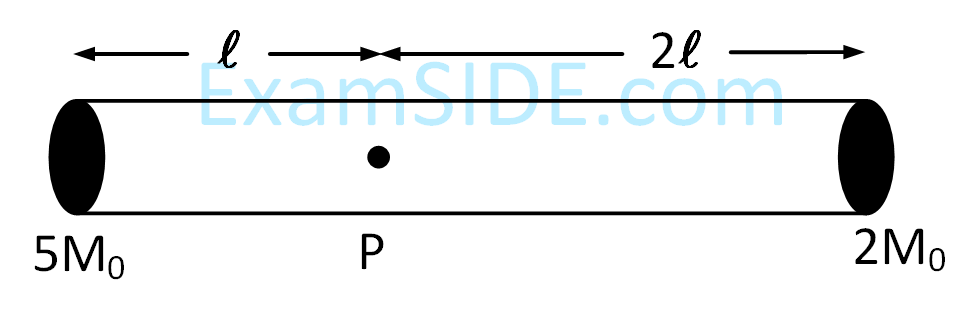
A rigid massless rod of length 3l has two masses attached at each end as shown in the figure. The rod is pivoted at point P on the horizontal axis (see figure). When released from initial horizontal position, its
instantaneous angular acceleration will be -

9
Half mole of an ideal monoatomic gas is heated at constant pressure of 1 atm from 20oC to 90oC. Work done
by gas is close to – (Gas constant R = 8.31 J/mol.K)
10
A parallel plate capacitor having capacitance 12 pF is charged by a battery to a potential difference of 10 V between its plates. The charging battery is now disconnected and a porcelain slab of dielectric constant 6.5 is slipped between the plates. The work done by the capacitor on the slab is :
11
A particle executes simple harmonic motion with an amplitude of 5 cm. When the particle is at 4 cm from the mean position, the magnitude of its velocity in SI units is equal to that of its acceleration. Then, its periodic time in seconds is -
12
An unknown metal of mass 192 g heated to a temperature of 100oC was immersed into a brass calorimeter of mass 128 g containing 240 g of water at a temperature of 8.4oC. Calculate the specific heat of the unknown metal if water temperature stabilizes at 21.5oC. (Specific heat of brass is 394 J kg–1 K–1)
13
The eye can be regarded as a single refracting surface. The radius of curvature of this surface is equal to that of cornea (7.8 mm). This surface separateds two media of refractive indices 1 and 1.34. Calculate the distance from the refracting surface at which a parallel beam of light will come to focus -
14
A cylindrical plastic bottle of negligible mass is filled with 310 ml of water and left floating in a pond with still water. If pressed downward slightly and released, it starts performing simple harmonic motion at angular frequency $$\omega $$. If the radius of the bottle is 2.5 cm then $$\omega $$ is close to – (density of water = 103 kg/m3).
15
A particle which is experiencing a force, given by $$\overrightarrow F = 3\widehat i - 12\widehat j,$$ undergoes a displacement of $$\overrightarrow d = 4\overrightarrow i $$ particle had a kinetic energy of 3 J at the beginning of the displacement, what is its kinetic energy at the end of the displacement ?
16
A hoop and a solid cylinder of same mass and radius are made of a permanent magnetic material with their magnetic moment parallel to their respective axes. But the magnetic moment of hoop is twice of solid cylinder. They are placed in a uniform magnetic field in such a manner that their magnetic moments make a small angle with the field. If the oscillation periods of hoop and cylinder are Th and Tc respectively, then -
17
Two forces P and Q, of magnitude 2F and 3F, respectively, are at an angle $$\theta $$ with each other. If the force Q is doubled, then their resultant also gets doubled. Then, the angle $$\theta $$ is -
18
Two kg of a monoatomic gas is at a pressure of 4 $$ \times $$ 104 N/m2. The density of the gas is 8 kg/m3. What is the order of energy of the gas due to its thermal motion ?
19
The electric field of a plane polarized electromagnetic wave in free space at time t = 0 is given by an expression $$\overrightarrow E \left( {x,y} \right) = 10\widehat j\cos \left[ {\left( {6x + 8z} \right)} \right].$$ The magnetic field $$\overrightarrow B $$(x,z, t) is given by $$-$$ (c is the velocity of light)
20
Two stars of masses 3 $$ \times $$ 1031 kg each, and at distance 2 $$ \times $$ 1011 m rotate in a plane about their common centre of mass O. A meteorite passes through O moving perpendicular to the star’s rotation plane. In order to escape from the gravitational field of this double star, the minimum speed that meteorite should have at O is - (Take Gravitational constant; G = 6.67 $$ \times $$ 10–11 Nm2 kg–2)
21
A current of 2 mA was passed through an unknown resistor which dissipated a power of 4.4 W. Dissipated power when an ideal power supply of 11 V is connected across it is -
22
The self induced emf of a coil is 25 volts. When the current in it is changed at uniform rate from 10 A to 25 A in 1s, the change in the energy of the inductance is -
23
The diameter and height of a cylinder are measured by a meter scale to be 12.6 $$ \pm $$ 0.1 cm and 34.2 $$ \pm $$ 0.1 cm, respectively. What will be the value of its volume in appropriate significant figures ?
24
Two vectors $$\overrightarrow A $$ and $$\overrightarrow B $$ have equal magnitudes. The magnitude of $$\left( {\overrightarrow A + \overrightarrow B } \right)$$ is 'n' times the magnitude of $$\left( {\overrightarrow A - \overrightarrow B } \right)$$ . The angle between $${\overrightarrow A }$$ and $${\overrightarrow B }$$ is -
25
Four equal point charges Q each are placed in the xy plane at (0, 2), (4, 2), (4, –2) and (0, –2). The work required to put a fifth charge Q at the origin of the coordinate system will be -
26
Consider the nuclear fission
Ne20 $$ \to $$ 2He4 + C12
Given that the binding energy/ nucleon of Ne20, He4 and C12 are, respectively, 8.03 MeV, 7.07 MeV and 7.86 MeV, identify the correct statement -
Ne20 $$ \to $$ 2He4 + C12
Given that the binding energy/ nucleon of Ne20, He4 and C12 are, respectively, 8.03 MeV, 7.07 MeV and 7.86 MeV, identify the correct statement -
27
A metal plate of area 1 $$ \times $$ 10–4 m2 is illuminated by a radiation of intensity 16 mW/m2. The work function of the metal is 5 eV. The energy of the incident photons is 10 eV and only 10% of it produces photo electrons.
The number of emitted photoelectrons per second and their maximum energy, respectively, will be