2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002JEE Main 2019 (Online) 10th April Morning Slot
Paper was held on Wed, Apr 10, 2019 3:30 AM
Chemistry
1
The oxoacid of sulphur that does not contain bond between sulphur atoms is
2
The increasing order of the reactivity of the following compounds towards electrophilic aromatic substitution reactions is :


3
The graph between $${\left| \psi \right|^2}$$ and r (radial distance) is shown below. This represents :


4
The major product of the following reaction is :


5
During the change of O2 to O2-
, the incoming electron goes to the orbital :
6
Three complexes,
[CoCl(NH3)5] 2+(I),
[Co(NH3)5H2O]3+ (II) and
[Co(NH3)6] 3+(III)
absorb light in the visible region. The correct order of the wavelength of light absorbed by them is :
[CoCl(NH3)5] 2+(I),
[Co(NH3)5H2O]3+ (II) and
[Co(NH3)6] 3+(III)
absorb light in the visible region. The correct order of the wavelength of light absorbed by them is :
7
The species that can have a trans-isomer is :
(en = ehane-1, 2-diamine, ox = oxalate)
(en = ehane-1, 2-diamine, ox = oxalate)
8
The major product of the following reaction is :


9
At room temperature, a dilute solution of urea is prepared by dissolving 0.60 of urea in 360 g of water. If the
vapour pressure of pure water at this temperature is 35 mm Hg, lowering of vapour pressure will be.
(molar mass of urea = 60 g mol–1)
10
The principle of column chromatography is :
11
A process will be spontaneous at all temperatures if :
12
At 300 K and 1 atmospheric pressure, 10 mL of a hydrocarbon required 55 mL of O2 for complete
combustion, and 40 mL of CO2 is formed. The formula of the hydrocarbon is :
13
Consider the following statements
(a) The pH of a mixture containing 400 mL of 0.1 M H2SO4 and 400 mL of 0.1 M NaOH will be approximately 1.3
(b) Ionic product of water is temperature dependent.
(c) A monobasic acid with Ka = 10–5 has pH = 5. The degree of dissociation of this acid is 50 %.
(d) The Le Chatelier's principle is not applicable to common-ion effect.
The correct statements are :
(a) The pH of a mixture containing 400 mL of 0.1 M H2SO4 and 400 mL of 0.1 M NaOH will be approximately 1.3
(b) Ionic product of water is temperature dependent.
(c) A monobasic acid with Ka = 10–5 has pH = 5. The degree of dissociation of this acid is 50 %.
(d) The Le Chatelier's principle is not applicable to common-ion effect.
The correct statements are :
14
A bacterial infection in an internal wound grows as N'(t) = N0 exp(t), where the time t is in hours. A does of antibiotic, taken orally, needs 1 hour to reach the wound. Once it reaches there, the bacterial population goes down as $${{dN} \over {dt}} = - 5{N^2}$$.
What will be the plot of $${{{N_0}} \over N}$$
vs. t after 1 hour?
15
The major product of the following reaction is :


16
Major products of the following reaction are :


17
The isoelectronic set of ions is :
18
Consider the statements S1 and S2
S1 : Conductivity always increases with decrease in the concentration of electrolyte.
S2 : Molar conductivity always increases with decrease in the concentration of electrolyte.
The correct option among the following is :
S1 : Conductivity always increases with decrease in the concentration of electrolyte.
S2 : Molar conductivity always increases with decrease in the concentration of electrolyte.
The correct option among the following is :
19
Consider the hydrated ions of Ti2+, V2+, Ti3+, and Sc3+. The correct order of their spin-only magnetic
moments is :
20
Amylopectin is compound of :
21
Ethylamine (C2H5NH2) can be obtained from N-ethylphatalimide on treatment with :
22
Increasing rate of SN1 reaction in the following compounds is :


Mathematics
1
Assume that each born child is equally likely to be a boy or a girl. If two families have two children each,
then the conditional probability that all children are girls given that at least two are girls is :
2
If $${\Delta _1} = \left| {\matrix{
x & {\sin \theta } & {\cos \theta } \cr
{ - \sin \theta } & { - x} & 1 \cr
{\cos \theta } & 1 & x \cr
} } \right|$$ and
$${\Delta _2} = \left| {\matrix{ x & {\sin 2\theta } & {\cos 2\theta } \cr { - \sin 2\theta } & { - x} & 1 \cr {\cos 2\theta } & 1 & x \cr } } \right|$$, $$x \ne 0$$ ;
then for all $$\theta \in \left( {0,{\pi \over 2}} \right)$$ :
$${\Delta _2} = \left| {\matrix{ x & {\sin 2\theta } & {\cos 2\theta } \cr { - \sin 2\theta } & { - x} & 1 \cr {\cos 2\theta } & 1 & x \cr } } \right|$$, $$x \ne 0$$ ;
then for all $$\theta \in \left( {0,{\pi \over 2}} \right)$$ :
3
If the length of the perpendicular from the point ($$\beta $$, 0, $$\beta $$) ($$\beta $$ $$ \ne $$ 0) to the line,
$${x \over 1} = {{y - 1} \over 0} = {{z + 1} \over { - 1}}$$ is $$\sqrt {{3 \over 2}} $$, then $$\beta $$ is equal to :
$${x \over 1} = {{y - 1} \over 0} = {{z + 1} \over { - 1}}$$ is $$\sqrt {{3 \over 2}} $$, then $$\beta $$ is equal to :
4
If y = y(x) is the solution of the differential equation
$${{dy} \over {dx}} = \left( {\tan x - y} \right){\sec ^2}x$$, $$x \in \left( { - {\pi \over 2},{\pi \over 2}} \right)$$,
such that y (0) = 0, then $$y\left( { - {\pi \over 4}} \right)$$ is equal to :
$${{dy} \over {dx}} = \left( {\tan x - y} \right){\sec ^2}x$$, $$x \in \left( { - {\pi \over 2},{\pi \over 2}} \right)$$,
such that y (0) = 0, then $$y\left( { - {\pi \over 4}} \right)$$ is equal to :
5
If a > 0 and z = $${{{{\left( {1 + i} \right)}^2}} \over {a - i}}$$, has magnitude $$\sqrt {{2 \over 5}} $$, then $$\overline z $$ is equal to :
6
If $$\alpha $$ and $$\beta $$ are the roots of the quadratic equation,
x2 + x sin $$\theta $$ - 2 sin $$\theta $$ = 0, $$\theta \in \left( {0,{\pi \over 2}} \right)$$, then
$${{{\alpha ^{12}} + {\beta ^{12}}} \over {\left( {{\alpha ^{ - 12}} + {\beta ^{ - 12}}} \right).{{\left( {\alpha - \beta } \right)}^{24}}}}$$ is equal to :
x2 + x sin $$\theta $$ - 2 sin $$\theta $$ = 0, $$\theta \in \left( {0,{\pi \over 2}} \right)$$, then
$${{{\alpha ^{12}} + {\beta ^{12}}} \over {\left( {{\alpha ^{ - 12}} + {\beta ^{ - 12}}} \right).{{\left( {\alpha - \beta } \right)}^{24}}}}$$ is equal to :
7
All the pairs (x, y) that satisfy the inequality
$${2^{\sqrt {{{\sin }^2}x - 2\sin x + 5} }}.{1 \over {{4^{{{\sin }^2}y}}}} \le 1$$
also satisfy the equation
$${2^{\sqrt {{{\sin }^2}x - 2\sin x + 5} }}.{1 \over {{4^{{{\sin }^2}y}}}} \le 1$$
also satisfy the equation
8
The region represented by| x – y | $$ \le $$ 2 and | x + y| $$ \le $$ 2 is bounded by a :
9
Let f(x) = ex – x and g(x) = x2 – x, $$\forall $$ x $$ \in $$ R. Then the set of all x $$ \in $$ R, where the function h(x) = (fog) (x) is increasing, is :
10
If a1, a2, a3, ............... an are in A.P. and a1 + a4 + a7 + ........... + a16 = 114, then a1 + a6 + a11 + a16 is equal to :
11
If for some x $$ \in $$ R, the frequency distribution of the marks obtained by 20 students in a test is :
then the mean of the marks is
| Marks | 2 | 3 | 5 | 7 |
|---|---|---|---|---|
| Frequency | (x + 1)2 | 2x - 5 | x2 - 3x | x |
then the mean of the marks is
12
The number of 6 digit numbers that can be formed using the digits 0, 1, 2, 5, 7 and 9 which are divisible by
11 and no digit is repeated is :
13
If the system of linear equations
x + y + z = 5
x + 2y + 2z = 6
x + 3y + $$\lambda $$z = $$\mu $$, ($$\lambda $$, $$\mu $$ $$ \in $$ R), has infinitely many solutions, then the value of $$\lambda $$ + $$\mu $$ is :
x + y + z = 5
x + 2y + 2z = 6
x + 3y + $$\lambda $$z = $$\mu $$, ($$\lambda $$, $$\mu $$ $$ \in $$ R), has infinitely many solutions, then the value of $$\lambda $$ + $$\mu $$ is :
14
Let A (3, 0, –1), B(2, 10, 6) and C(1, 2, 1) be the vertices of a triangle and M be the midpoint of AC. If G
divides BM in the ratio, 2 : 1, then cos ($$\angle $$GOA) (O being the origin) is equal to :
15
Let f : R $$ \to $$ R be differentiable at c $$ \in $$ R and f(c) = 0. If g(x) = |f(x)| , then at x = c, g is :
16
The value of $$\int\limits_0^{2\pi } {\left[ {\sin 2x\left( {1 + \cos 3x} \right)} \right]} dx$$,
where [t] denotes the greatest integer function is :
where [t] denotes the greatest integer function is :
17
If $$\int {{{dx} \over {{{\left( {{x^2} - 2x + 10} \right)}^2}}}} = A\left( {{{\tan }^{ - 1}}\left( {{{x - 1} \over 3}} \right) + {{f\left( x \right)} \over {{x^2} - 2x + 10}}} \right) + C$$
where C is a constant of integration then :
where C is a constant of integration then :
18
If$$f(x) = \left\{ {\matrix{
{{{\sin (p + 1)x + \sin x} \over x}} & {,x < 0} \cr
q & {,x = 0} \cr
{{{\sqrt {x + {x^2}} - \sqrt x } \over {{x^{{\raise0.5ex\hbox{$\scriptstyle 3$}
\kern-0.1em/\kern-0.15em
\lower0.25ex\hbox{$\scriptstyle 2$}}}}}}} & {,x > 0} \cr
} } \right.$$
is continuous at x = 0, then the ordered pair (p, q) is equal to
is continuous at x = 0, then the ordered pair (p, q) is equal to
19
If a directrix of a hyperbola centred at the origin and passing through the point (4, –2$$\sqrt 3 $$ ) is 5x = 4$$\sqrt 5 $$ and
its eccentricity is e, then :
20
If $$\mathop {\lim }\limits_{x \to 1} {{{x^4} - 1} \over {x - 1}} = \mathop {\lim }\limits_{x \to k} {{{x^3} - {k^3}} \over {{x^2} - {k^2}}}$$, then k is :
21
Let f(x) = x2
, x $$ \in $$ R. For any A $$ \subseteq $$ R, define g (A) = { x $$ \in $$ R : f(x) $$ \in $$ A}. If S = [0,4], then which one of the
following statements is not true ?
Physics
1
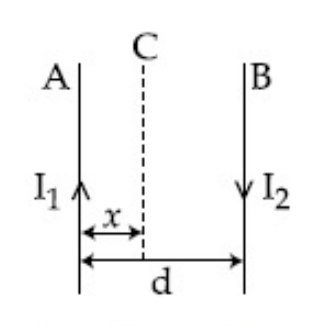
Two wires A & B are carrying currents I1 & I2
as shown in the figure. The separation between
them is d. A third wire C carrying a current I
is to be kept parallel to them at a distance x from
A such that the net force acting on it is zero.
The possible values of x are :

2
A 25 × 10–3 m3 volume cylinder is filled with
1 mol of O2 gas at room temperature (300K).
The molecular diameter of O2, and its root
mean square speed, are found to be 0.3 nm, and
200 m/s, respectively. What is the average
collision rate (per second) for an O2 molecule ?
3
In a photoelectric effect experiment the
threshold wavelength of the light is 380 nm. If
the wavelentgh of incident light is 260 nm, the
maximum kinetic energy of emitted electrons
will be:
Given E (in eV) = 1237/$$\lambda $$ (in nm)
Given E (in eV) = 1237/$$\lambda $$ (in nm)
4
A transformer consisting of 300 turns in the
primary and 150 turns in the secondary gives
output power of 2.2 kW. If the current in the
secondary coil is 10A, then the input voltage
and current in the primary coil are :
5
In an experiment, the resistance of a material
is plotted as a function of temperature (in some
range). As shown in the figure, it is a straight
line. One may conclude that :


6
A ray of light AO in vacuum is incident on a
glass slab at angle 60° and refracted at angle 30°
along OB as shown in the figure. The optical path
length of light ray from A to B is:


7
n moles of an ideal gas with constant volume
heat capcity CV undergo an isobaric expansion
by certain volume. The ratio of the work done
in the process, to the heat supplied is :
8
A proton, an electron, and a Helium nucleus,
have the same energy. They are in circular
orbits in a plane due to magnetic field
perpendicualr to the plane. Let rp, re and rHe be
their respective radii, then
9
A particle of mass m is moving along a
trajectory given by
x = x0 + a cos$$\omega $$1t
y = y0 + b sin$$\omega $$2t
The torque, acting on the particle about the origin, at t = 0 is :
x = x0 + a cos$$\omega $$1t
y = y0 + b sin$$\omega $$2t
The torque, acting on the particle about the origin, at t = 0 is :
10
The electric field of a plane electromagnetic
wave is given by
$$\overrightarrow E = {E_0}\widehat i\cos (kz)cos(\omega t)$$
The corresponding magnetic field $$\overrightarrow B $$ is then given by
$$\overrightarrow E = {E_0}\widehat i\cos (kz)cos(\omega t)$$
The corresponding magnetic field $$\overrightarrow B $$ is then given by
11
Two coaxial discs, having moments of inertia
I1 and I1/2, are rotating with respective angular
velocities $$\omega $$1 and
$$\omega $$1/2
, about their common axis.
They are brought in contact with each other and
thereafter they rotate with a common angular
velocity. If Ef and Ei are the final and initial total
energies, then (Ef - Ei) is:
12
A thin disc of mass M and radius R has mass
per unit area $$\sigma $$(r) = kr2 where r is the distance
from its centre. Its moment of inertia about an
axis going through its centre of mass and
perpendicular to its plane is :
13
A cylinder with fixed capacity of 67.2 lit
contains helium gas at STP. The amount of heat
needed to raise the temperature of the gas by
20°C is : [Given that R = 8.31 J mol–1 K–1]
14
A ball is thrown upward with an initial velocity
V0 from the surface of the earth. The motion
of the ball is affected by a drag force equal to
m$$\gamma $$u2 (where m is mass of the ball, u is its
instantaneous velocity and $$\gamma $$ is a constant).
Time taken by the ball to rise to its zenith is :
15
One plano-convex and one plano-concave lens
of same radius of curvature 'R' but of different
materials are joined side by side as shown in
the figure. If the refractive index of the material
of 1 is $$\mu $$1 and that of 2 is $$\mu $$2, then the focal
length of the combination is :


16
In a meter bridge experiment, the circuit
diagram and the corresponding observation
table are shown in figure

Which of the readings is inconsistent?

| SI. No. | R($$\Omega $$) | l(cm) |
|---|---|---|
| 1. | 1000 | 60 |
| 2. | 100 | 13 |
| 3. | 10 | 1.5 |
| 4. | 1 | 1.0 |
17
A current of 5 A passes through a copper
conductor (resistivity = 1.7 × 10–8 $$\Omega $$m) of radius
of cross-section 5 mm. Find the mobility of the
charges if their drift velocity is 1.1 × 10–3 m/s.
18
Figure shows charge (q) versus voltage (V)
graph for series and parallel combination of two
given capacitors. The capacitances are :


19
Two particles, of masses M and 2M, moving,
as shown, with speeds of 10 m/s and 5 m/s,
collide elastically at the origin. After the
collision, they move along the indicated
directions with speeds u1 and u2, respectively.
The values of u1 and u2 are nearly :


20
The ratio of surface tensions of mercury and
water is given to be 7.5 while the ratio of thier
densities is 13.6. Their contact angles, with
glass, are close to 135° and 0°, respectively. It
is observed that mercury gets depressed by an
amount h in a capillary tube of radius r1, while
water rises by the same amount h in a capillary
tube of radius r2. The ratio, (r1/r2), is then close
to :
21
The value of acceleration due to gravity at
Earth's surface is 9.8 ms–2. The altitude above
its surface at which the acceleration due to
gravity decreases to 4.9 ms–2, is close to :
(Radius of earth = 6.4 × 106 m)
22
A uniformly charged ring of radius 3a and total
charge q is placed in xy-plane centred at origin.
A point charge q is moving towards the ring
along the z-axis and has speed u at z = 4a. The
minimum value of u such that it crosses the
origin is :
23
In the given circuit, an ideal voltmeter
connected across the 10$$\Omega $$ resistance reads 2V.
The internal resistance r, of each cell is:


24
A moving coil galvanometer allows a full scale
current of 10–4 A. A series resistance of 2 M$$\Omega $$
is required to convert the above galvanometer
into a voltmeter of range 0-5 V. Therefore the
value of shunt resistance required to convert the
above galvanometer into an ammeter of range
0.10 mA is :