2025
JEE Main 2025 (Online) 8th April Evening ShiftJEE Main 2025 (Online) 7th April Evening ShiftJEE Main 2025 (Online) 7th April Morning ShiftJEE Main 2025 (Online) 4th April Evening ShiftJEE Main 2025 (Online) 4th April Morning ShiftJEE Main 2025 (Online) 3rd April Evening ShiftJEE Main 2025 (Online) 3rd April Morning ShiftJEE Main 2025 (Online) 2nd April Evening ShiftJEE Main 2025 (Online) 2nd April Morning ShiftJEE Main 2025 (Online) 29th January Evening ShiftJEE Main 2025 (Online) 29th January Morning ShiftJEE Main 2025 (Online) 28th January Evening ShiftJEE Main 2025 (Online) 28th January Morning ShiftJEE Main 2025 (Online) 24th January Evening ShiftJEE Main 2025 (Online) 24th January Morning ShiftJEE Main 2025 (Online) 23rd January Evening ShiftJEE Main 2025 (Online) 23rd January Morning ShiftJEE Main 2025 (Online) 22nd January Evening ShiftJEE Main 2025 (Online) 22nd January Morning Shift2024
JEE Main 2024 (Online) 9th April Evening ShiftJEE Main 2024 (Online) 9th April Morning ShiftJEE Main 2024 (Online) 8th April Evening ShiftJEE Main 2024 (Online) 8th April Morning ShiftJEE Main 2024 (Online) 6th April Evening ShiftJEE Main 2024 (Online) 6th April Morning ShiftJEE Main 2024 (Online) 5th April Evening ShiftJEE Main 2024 (Online) 5th April Morning ShiftJEE Main 2024 (Online) 4th April Evening ShiftJEE Main 2024 (Online) 4th April Morning ShiftJEE Main 2024 (Online) 1st February Evening ShiftJEE Main 2024 (Online) 1st February Morning ShiftJEE Main 2024 (Online) 31st January Evening ShiftJEE Main 2024 (Online) 31st January Morning ShiftJEE Main 2024 (Online) 30th January Evening ShiftJEE Main 2024 (Online) 30th January Morning ShiftJEE Main 2024 (Online) 29th January Evening ShiftJEE Main 2024 (Online) 29th January Morning ShiftJEE Main 2024 (Online) 27th January Evening ShiftJEE Main 2024 (Online) 27th January Morning Shift2023
JEE Main 2023 (Online) 15th April Morning ShiftJEE Main 2023 (Online) 13th April Evening ShiftJEE Main 2023 (Online) 13th April Morning ShiftJEE Main 2023 (Online) 12th April Morning ShiftJEE Main 2023 (Online) 11th April Evening ShiftJEE Main 2023 (Online) 11th April Morning ShiftJEE Main 2023 (Online) 10th April Evening ShiftJEE Main 2023 (Online) 10th April Morning ShiftJEE Main 2023 (Online) 8th April Evening ShiftJEE Main 2023 (Online) 8th April Morning ShiftJEE Main 2023 (Online) 6th April Evening ShiftJEE Main 2023 (Online) 6th April Morning ShiftJEE Main 2023 (Online) 1st February Evening ShiftJEE Main 2023 (Online) 1st February Morning ShiftJEE Main 2023 (Online) 31st January Evening ShiftJEE Main 2023 (Online) 31st January Morning ShiftJEE Main 2023 (Online) 30th January Evening ShiftJEE Main 2023 (Online) 30th January Morning ShiftJEE Main 2023 (Online) 29th January Evening ShiftJEE Main 2023 (Online) 29th January Morning ShiftJEE Main 2023 (Online) 25th January Evening ShiftJEE Main 2023 (Online) 25th January Morning ShiftJEE Main 2023 (Online) 24th January Evening ShiftJEE Main 2023 (Online) 24th January Morning Shift2022
JEE Main 2022 (Online) 29th July Evening ShiftJEE Main 2022 (Online) 29th July Morning ShiftJEE Main 2022 (Online) 28th July Evening ShiftJEE Main 2022 (Online) 28th July Morning ShiftJEE Main 2022 (Online) 27th July Evening ShiftJEE Main 2022 (Online) 27th July Morning ShiftJEE Main 2022 (Online) 26th July Evening ShiftJEE Main 2022 (Online) 26th July Morning ShiftJEE Main 2022 (Online) 25th July Evening ShiftJEE Main 2022 (Online) 25th July Morning ShiftJEE Main 2022 (Online) 30th June Morning ShiftJEE Main 2022 (Online) 29th June Evening ShiftJEE Main 2022 (Online) 29th June Morning ShiftJEE Main 2022 (Online) 28th June Evening ShiftJEE Main 2022 (Online) 28th June Morning ShiftJEE Main 2022 (Online) 27th June Evening ShiftJEE Main 2022 (Online) 27th June Morning ShiftJEE Main 2022 (Online) 26th June Evening ShiftJEE Main 2022 (Online) 26th June Morning ShiftJEE Main 2022 (Online) 25th June Evening ShiftJEE Main 2022 (Online) 25th June Morning ShiftJEE Main 2022 (Online) 24th June Evening ShiftJEE Main 2022 (Online) 24th June Morning Shift2021
JEE Main 2021 (Online) 1st September Evening ShiftJEE Main 2021 (Online) 31st August Evening ShiftJEE Main 2021 (Online) 31st August Morning ShiftJEE Main 2021 (Online) 27th August Evening ShiftJEE Main 2021 (Online) 27th August Morning ShiftJEE Main 2021 (Online) 26th August Evening ShiftJEE Main 2021 (Online) 26th August Morning ShiftJEE Main 2021 (Online) 27th July Evening ShiftJEE Main 2021 (Online) 27th July Morning ShiftJEE Main 2021 (Online) 25th July Evening ShiftJEE Main 2021 (Online) 25th July Morning ShiftJEE Main 2021 (Online) 22th July Evening ShiftJEE Main 2021 (Online) 20th July Evening ShiftJEE Main 2021 (Online) 20th July Morning ShiftJEE Main 2021 (Online) 18th March Evening ShiftJEE Main 2021 (Online) 18th March Morning ShiftJEE Main 2021 (Online) 17th March Evening ShiftJEE Main 2021 (Online) 17th March Morning ShiftJEE Main 2021 (Online) 16th March Evening ShiftJEE Main 2021 (Online) 16th March Morning ShiftJEE Main 2021 (Online) 26th February Evening ShiftJEE Main 2021 (Online) 26th February Morning ShiftJEE Main 2021 (Online) 25th February Evening ShiftJEE Main 2021 (Online) 25th February Morning ShiftJEE Main 2021 (Online) 24th February Evening ShiftJEE Main 2021 (Online) 24th February Morning Shift2020
JEE Main 2020 (Online) 6th September Evening SlotJEE Main 2020 (Online) 6th September Morning SlotJEE Main 2020 (Online) 5th September Evening SlotJEE Main 2020 (Online) 5th September Morning SlotJEE Main 2020 (Online) 4th September Evening SlotJEE Main 2020 (Online) 4th September Morning SlotJEE Main 2020 (Online) 3rd September Evening SlotJEE Main 2020 (Online) 3rd September Morning SlotJEE Main 2020 (Online) 2nd September Evening SlotJEE Main 2020 (Online) 2nd September Morning SlotJEE Main 2020 (Online) 9th January Evening SlotJEE Main 2020 (Online) 9th January Morning SlotJEE Main 2020 (Online) 8th January Evening SlotJEE Main 2020 (Online) 8th January Morning SlotJEE Main 2020 (Online) 7th January Evening SlotJEE Main 2020 (Online) 7th January Morning Slot2019
JEE Main 2019 (Online) 12th April Evening SlotJEE Main 2019 (Online) 12th April Morning SlotJEE Main 2019 (Online) 10th April Evening SlotJEE Main 2019 (Online) 10th April Morning SlotJEE Main 2019 (Online) 9th April Evening SlotJEE Main 2019 (Online) 9th April Morning SlotJEE Main 2019 (Online) 8th April Evening SlotJEE Main 2019 (Online) 8th April Morning SlotJEE Main 2019 (Online) 12th January Evening SlotJEE Main 2019 (Online) 12th January Morning SlotJEE Main 2019 (Online) 11th January Evening SlotJEE Main 2019 (Online) 11th January Morning SlotJEE Main 2019 (Online) 10th January Evening SlotJEE Main 2019 (Online) 10th January Morning SlotJEE Main 2019 (Online) 9th January Evening SlotJEE Main 2019 (Online) 9th January Morning Slot2018
JEE Main 2018 (Online) 16th April Morning SlotJEE Main 2018 (Offline)JEE Main 2018 (Online) 15th April Evening SlotJEE Main 2018 (Online) 15th April Morning Slot2017
JEE Main 2017 (Online) 9th April Morning SlotJEE Main 2017 (Online) 8th April Morning SlotJEE Main 2017 (Offline)2016
JEE Main 2016 (Online) 10th April Morning SlotJEE Main 2016 (Online) 9th April Morning SlotJEE Main 2016 (Offline)2015
JEE Main 2015 (Offline)2014
JEE Main 2014 (Offline)2013
JEE Main 2013 (Offline)2012
AIEEE 20122011
AIEEE 20112010
AIEEE 20102009
AIEEE 20092008
AIEEE 20082007
AIEEE 20072006
AIEEE 20062005
AIEEE 20052004
AIEEE 20042003
AIEEE 20032002
AIEEE 2002JEE Main 2019 (Online) 10th April Evening Slot
Paper was held on Wed, Apr 10, 2019 9:30 AM
Chemistry
1
The increasing order of nucleophilicity of the following nucleophiles is :
(a) CH3CO2$$-$$ (b) H2O
(c) CH3SO3$$-$$ (d) $$\mathop O\limits^ - H$$
(a) CH3CO2$$-$$ (b) H2O
(c) CH3SO3$$-$$ (d) $$\mathop O\limits^ - H$$
2
The major product 'Y' in the following reaction is :


3
Which one of the following graphs between molar conductivity ($${\Lambda _m}$$) versus $$\sqrt C $$ is correct ?
4
The crystal field stabilization energy (CFSE) of [Fe(H2O)6]Cl2 and K2[NiCl4] respectively, are :
5
Which of these factors does not govern the stability of a conformation in acyclic compounds?
6
The pH of a 0.02 M NH4Cl solution will be :
[given Kb (NH4OH) = 10–5 and log 2 = 0.301]
[given Kb (NH4OH) = 10–5 and log 2 = 0.301]
7
The difference between $$\Delta $$H and $$\Delta $$U ($$\Delta $$H – $$\Delta $$U), when the combustion of one mole of heptane(l) is carried
out at a temperature T, is equal to :
8
1 g of a non-volatile non-electrolyte solute is dissolved in 100 g of two different solvents A and B whose
ebullioscopic constants are in the ratio of 1 : 5. The ratio of the elevation in their boiling points, $${{\Delta {T_b}(A)} \over {\Delta {T_b}(B)}}$$, is :
9
The minimum amount of O2(g) consumed per gram of reactant is for the reaction :
(Given atomic mass : Fe = 56, O = 16, Mg = 24, P = 31, C = 12, H = 1)
(Given atomic mass : Fe = 56, O = 16, Mg = 24, P = 31, C = 12, H = 1)
10
For the reaction,
2SO2(g) + O2(g) = 2SO3(g), $$\Delta $$H = –57.2 kJ mol–1 and KC = 1.7 × 1016
Which of the following statement is incorrect ?
2SO2(g) + O2(g) = 2SO3(g), $$\Delta $$H = –57.2 kJ mol–1 and KC = 1.7 × 1016
Which of the following statement is incorrect ?
11
The correct order of the first ionization enthalpies is :
12
The ratio of the shortest wavelength of two spectral series of hydrogen spectrum is found to be about 9. The
spectral series are :
13
Compound A(C9H10O) shows positive iodoform test. Oxidation of A with KMnO4/KOH given acid
B(C8H6O4). Anhydride of B is used for the preparation of phenolphthalein. Compound A is:
14
The major product 'Y' in the following reactions is :


15
For the reaction of H2 with I2, the rate constant is 2.5 × 10–4 dm3
mol–1s–1
at 327°C and 1.0 dm3
mol–1
at
527°C. The activation energy for the reaction, in kJ mole–1
is : (R = 8.314 JK–1
mol–1
)
16
The INCORRECT statement is :
17
In chromatography, which of the following statements is incorrect for Rf ?
18
Number of stereo centers present in linear and cyclic structures of glucose are respectively :
19
The highest possible oxidation states of uranium and plutonium, respectively are :
20
The noble gas that does not occur in the atmosphere is :
21
Which of the following is not a correct method of the preparation of benzylamine from cyanobenzene ?
22
The major product obtained in the given reaction is :


Mathematics
1
Let f(x) = loge(sin x), (0 < x < $$\pi $$) and g(x) = sin–1
(e–x
), (x $$ \ge $$ 0). If $$\alpha $$ is a positive real number such that
a = (fog)'($$\alpha $$) and b = (fog)($$\alpha $$), then :
2
A spherical iron ball of radius 10 cm is coated with a layer of ice of uniform thickness that melts at a rate of
50 cm3
/min. When the thickness of the ice is 5 cm, then the rate at which the thickness (in cm/min) of the ice
decreases, is :
3
The number of real roots of the equation
5 + |2x – 1| = 2x (2x – 2) is
5 + |2x – 1| = 2x (2x – 2) is
4
If $$\mathop {\lim }\limits_{x \to 1} {{{x^2} - ax + b} \over {x - 1}} = 5$$, then a + b is equal to :
5
Minimum number of times a fair coin must be tossed so that the probability of getting at least one head is
more than 99% is :
6
If $$\int {{x^5}} {e^{ - {x^2}}}dx = g\left( x \right){e^{ - {x^2}}} + c$$, where c is a constant of integration, then $$g$$(–1) is equal to :
7
Let a1, a2, a3,......be an A.P. with a6 = 2. Then the common difference of this A.P., which maximises the
product a1a4a5, is :
8
The distance of the point having position vector $$ - \widehat i + 2\widehat j + 6\widehat k$$
from the straight line passing through the point
(2, 3, – 4) and parallel to the vector, $$6\widehat i + 3\widehat j - 4\widehat k$$ is :
9
If $${\cos ^{ - 1}}x - {\cos ^{ - 1}}{y \over 2} = \alpha $$,where –1 $$ \le $$ x $$ \le $$ 1, – 2 $$ \le $$ y $$ \le $$ 2, x $$ \le $$ $${y \over 2}$$
, then for all x, y, 4x2
– 4xy cos $$\alpha $$ + y2
is equal
to :
10
If both the mean and the standard deviation of 50 observations x1, x2,..., x50 are equal to 16, then the mean of (x1 – 4)2
, (x2 – 4)2
,....., (x50 – 4)2
is :
11
Lines are drawn parallel to the line 4x – 3y + 2 = 0, at a distance
$${3 \over 5}$$
from the origin. Then which one of the
following points lies on any of these lines ?
12
Let y = y(x) be the solution of the differential equation,
$${{dy} \over {dx}} + y\tan x = 2x + {x^2}\tan x$$, $$x \in \left( { - {\pi \over 2},{\pi \over 2}} \right)$$, such that y(0) = 1. Then :
$${{dy} \over {dx}} + y\tan x = 2x + {x^2}\tan x$$, $$x \in \left( { - {\pi \over 2},{\pi \over 2}} \right)$$, such that y(0) = 1. Then :
13
Let $$a$$, b and c be in G.P. with common ratio r, where $$a$$ $$ \ne $$ 0 and 0 < r $$ \le $$ $${1 \over 2}$$
. If 3$$a$$, 7b and 15c are the first three
terms of an A.P., then the 4th term of this A.P. is :
14
Suppose that 20 pillars of the same height have been erected along the boundary of a circular stadium. If the
top of each pillar has been connected by beams with the top of all its non-adjacent pillars, then the total
number of beams is :
15
The integral $$\int\limits_{\pi /6}^{\pi /3} {{{\sec }^{2/3}}} x\cos e{c^{4/3}}xdx$$ is equal to :
16
The area (in sq.units) of the region bounded by the curves y = 2x
and y = |x + 1|, in the first quadrant is :
17
If z and w are two complex numbers such that |zw| = 1 and arg(z) – arg(w) = $${\pi \over 2}$$
, then :
18
The sum of the real roots of the equation
$$\left| {\matrix{ x & { - 6} & { - 1} \cr 2 & { - 3x} & {x - 3} \cr { - 3} & {2x} & {x + 2} \cr } } \right| = 0$$, is equal to :
$$\left| {\matrix{ x & { - 6} & { - 1} \cr 2 & { - 3x} & {x - 3} \cr { - 3} & {2x} & {x + 2} \cr } } \right| = 0$$, is equal to :
19
If 5x + 9 = 0 is the directrix of the hyperbola 16x2
– 9y2
= 144, then its corresponding focus is :
20
The smallest natural number n, such that the coefficient of x in the expansion of $${\left( {{x^2} + {1 \over {{x^3}}}} \right)^n}$$ is nC23, is :
21
Let $$\lambda $$ be a real number for which the system of linear equations x + y + z = 6, 4x + $$\lambda $$y – $$\lambda $$z = $$\lambda $$ – 2,
3x + 2y – 4z = – 5 has infinitely many solutions. Then $$\lambda $$ is a root of the quadratic equation:
Physics
1
A submarine experiences a pressure of 5.05 × 106
Pa at a depth of d1 in a sea. When it goes further to a depth
of d2, it experiences a pressure of 8.08 × 106
Pa. Then d2 –d1 is approximately (density of water = 103
kg/m3
and acceleration due to gravity = 10 ms–2
) :
2
A plane is inclined at an angle $$\alpha $$ = 30° with respect to the horizontal. A particle is projected with a speed u =
2 ms–1
, from the base of the plane, making an angle $$\theta $$ = 15° with respect to the plane as shown in the figure.
the distance from the base, at which the particle hits the plane is close to :
(Take g = 10 ms –2)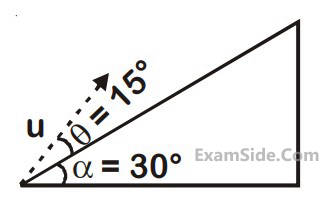
(Take g = 10 ms –2)

3
The time dependence of the position of a particle of mass m = 2 is given by $$\overrightarrow r \left( t \right) = 2t\widehat i - 3{t^2}\widehat j$$
. Its angular
momentum, with respect to the origin, at time t = 2 is
4
The magnitude of the magnetic field at the centre of an equilateral triangular loop of side 1 m which is
carrying a current of 10 A is :
[Take $$\mu $$0 = 4$$\pi $$ × 10–7 NA–2]
[Take $$\mu $$0 = 4$$\pi $$ × 10–7 NA–2]
5
Two blocks A and B of masses mA = 1 kg and mB = 3 kg are kept on the table as shown in figure. The coefficient of friction between A and B is 0.2 and between B and the surface of the table is also 0.2. The maximum force F that can be applied on B horizontally, so that the block A does not slide over the block B is :
[Take g = 10 m/s2]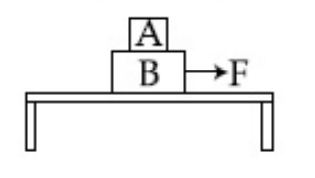
[Take g = 10 m/s2]

6
Light is incident normally on a completely absorbing surface with an energy flux of 25 W cm–2. If the surface
has an area of 25 cm2, the momentum transferred to the surface in 40 min time duration will be :
7
A solid sphere of mass M and radius R is divided into two unequal parts. The first part has a mass of $${{7M} \over 8}$$
and is converted into a uniform disc of radius 2R. The second part is converted into a uniform solid sphere.
Let I1 be the moment of inertia of the disc about its axis and I2 be the moment of inertia of the new sphere
about its axis. The ratio I1/I2 is given by :
8
In Li+ +, electron in first Bohr orbit is excited to a level by a radiation of wavelength $$\lambda $$. When the ion gets
deexcited to the ground state in all possible ways (including intermediate emissions), a total of six spectral
lines are observed. What is the value of $$\lambda $$?
(Given : H = 6.63 × 10–34 Js; c = 3 × 108 ms –1)
(Given : H = 6.63 × 10–34 Js; c = 3 × 108 ms –1)
9
In a Young's double slit experiment, the ratio of the slit's width is 4 : 1. The ratio of the intensity of maxima to minima, close to the central fringe on the screen, will be :
10
A spaceship orbits around a planet at a height of 20 km from its surface. Assuming that only gravitational
field of the planet acts on the spaceship, what will be the number of complete revolutions made by the
spaceship in 24 hours around the planet?
[Given ; Mass of planet = 8 × 1022 kg, Radius of planet = 2 × 106 m, Gravitational constant G = 6.67 × 10–11 Nm2 /kg2]
[Given ; Mass of planet = 8 × 1022 kg, Radius of planet = 2 × 106 m, Gravitational constant G = 6.67 × 10–11 Nm2 /kg2]
11
The correct figure that shows, schematically, the wave pattern produced by superposition of two waves of
frequencies 9 Hz and 11 Hz, is :
12
In an experiment, brass and steel wires of length 1 m each with areas of cross section 1mm2
are used. The
wires are connected in series and one end of the combined wire is connected to a rigid support and other end
is subjected to elongation. The stress required to produce a net elongation of 0.2 mm is,
[Given, the Young's Modulus for steel and brass are, respectively, 120 × 109
N/m2
and 60 × 109
N/m2]
13
A square loop is carrying a steady current I and the magnitude of its magnetic dipole moment is m. if this
square loop is changed to a circular loop and it carries the same current, the magnitude of the magnetic dipole
moment of circular loop will be:
14
Water from a tap emerges vertically downwards with an initial speed of 1.0 ms–1
. The cross-sectional area of
the tap is 10–4 m2. Assume that the pressure is constant throughout the stream of water and that the flow is streamlined. The cross-sectional area of the stream, 0.15 m below the tap would be : (Take g = 10 ms–2)
15
One mole of ideal gas passes through a process where pressure and volume obey the relation
$$P = {P_0}\left[ {1 - {1 \over 2}{{\left( {{{{V_0}} \over V}} \right)}^2}} \right]$$.
Here P0 and V0 are constants. Calculate the change in the temperature of the gas if its
volume changes form V0 to 2V0
16
Space between two concentric conducting spheres of radii a and b (b > a) is filled with a medium of
resistivity $$\rho $$. The resistance between the two spheres will be :
17
The figure represents a voltage regulator circuit using a Zener diode. The breakdown voltage of the Zener
diode is 6 V and the load resistance is, RL = 4k$$\Omega $$. The series resistance of the circuit is Ri
= 1 k$$\Omega $$. If the
battery voltage VB varies from 8 V to 16 V, what are the minimum and maximum values of the current
through Zener diode?


18
A metal coin of mass 5 g and radius 1 cm is fixed to a thin stick AB of negligible mass as shown in the
figure. The system is initially at rest. The constant torque, that will make the system rotate about AB at 25
rotations per second in 5s, is close to :
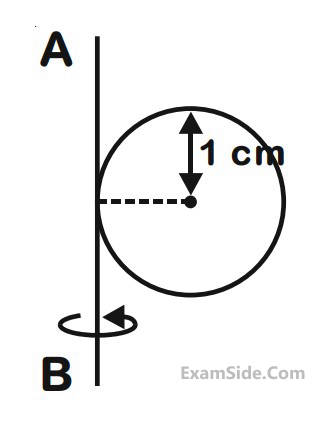

19
A coil of self inductance 10 mH and resistance 0.1 $$\Omega $$ is connected through a switch to a battery of internal
resistance 0.9 $$\Omega $$. After the switch is closed, the time taken for the current to attain 80% of the saturation
value is: [take ln 5 = 1.6]
20
A bullet of mass 20 g has an initial speed of 1 ms–1
, just before it starts penetrating a mud wall of thickness
20 cm. If the wall offers a mean resistance of 2.5 × 10–2 N, the speed of the bullet after emerging from the
other side of the wall is close to :
21
In the formula X = 5YZ2
, X and Z have dimensions of capacitance and magnetic field, respectively. What are
the dimensions of Y in SI units?
22
In free space, a particle A of charge 1$$\mu $$C is held fixed at a point P. Another particle B of the same charge and
mass 4$$\mu $$g is kept at a distance of 1 mm from P. If B is released, then its velocity at a distance of 9 mm from P
is :
$$\left[ {Take\,{1 \over {4\pi { \in _0}}} = 9 \times {{10}^9}N{m^2}{C^{ - 2}}} \right]$$
23
When heat Q is supplied to a diatomic gas of rigid molecules, at constant volume its temperature increases by
$$\Delta $$T. the heat required to produce the same change in temperature, at a constant pressure is :
24
The elastic limit of brass is 379 MPa. What should be the minimum diameter of a brass rod if it is to support
a 400 N load without exceeding its elastic limit?
25
A 2 mW laser operates at wavelength of 500 nm. The number of photons that will be emitted per second is :
[Given Planck's constant h = 6.6 × 10–34 Js, speed of light c = 3.0 × 108
m/s]
26
A cubical block of side 0.5 m floats on water with 30% of its volume under water. What is the maximum
weight that can be put on the block without fully submerging it under water? [Take, density of water = 103
kg/m3]
27
The graph shows how the magnification m produced by a thin lens varies with image distance v. What is the focal length of the lens used?


28
A simple pendulum of length L is placed between the plates of a parallel plate capacitor having electric field
E, as shown in figure. Its bob has mass m and charge q. The time period of the pendulum is given by :

