Thermodynamics
1
GATE ME 2008
MCQ (Single Correct Answer)
+2
-0.6
In the figure shown, the system is a pure substance kept in a piston- cylinder arrangement. The system is initially a two- phase mixture containing $$1$$ $$kg$$ of liquid and $$0.03$$ $$kg$$ of vapour at a pressure of $$100kPa.$$ Initially, the piston rests on a set of stops, as shown in the figure. A pressure of $$200kPa$$ is required to exactly balance the weight of the piston and the outside atmospheric pressure. Heat transfer takes place into the system until its volume increases by $$50\% $$. Heat transfer to the system occurs in such a manner that the piston, when allowed to move, does so in a very slow (quasi-static / quasi-equilibrium) process. The thermal reservoir from which heat is transferred to the system as a temperature of $${400^ \circ }C$$. Average temperature of the system boundary can be taken as $${175^ \circ }C.$$ Heat transfer to the system is $$1kJ$$, during which its entropy increases by $$10$$ $$J/K.$$



Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

The net entropy generation (considering the system and the thermal reservoir together) during the process is closest to
2
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
Given below is an extract from steam tables.
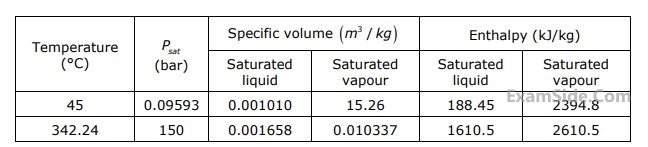

Specific enthalpy of water in $$kJ/kg$$ at $$150$$ bar and $$ {45^ \circ }C$$ is
3
GATE ME 2005
MCQ (Single Correct Answer)
+2
-0.6
The following table of properties was printed out for saturated liquid and saturated vapour of ammonia. The titles for only the first two columns are available. All that we know is that the other columns (columns $$3$$ to $$8$$) contain data on specific properties, namely, internal energy $$(kJ/kg),$$ enthalpy $$(kJ/kg)$$ and entropy $$(kJ/kg.K).$$


The specific enthalpy data are in columns
4
GATE ME 2005
MCQ (Single Correct Answer)
+2
-0.6
The following table of properties was printed out for saturated liquid and saturated vapour of ammonia. The titles for only the first two columns are available. All that we know is that the other columns (columns $$3$$ to $$8$$) contain data on specific properties, namely, internal energy $$(kJ/kg),$$ enthalpy $$(kJ/kg)$$ and entropy $$(kJ/kg.K).$$


When saturated liquid at $${40^ \circ }C$$ is throttled to $$ - {20^ \circ }C,$$ the quality at exit will be
Questions Asked from Marks 2
GATE ME Subjects
Engineering Mechanics
Machine Design
Strength of Materials
Heat Transfer
Production Engineering
Industrial Engineering
Turbo Machinery
Theory of Machines
Engineering Mathematics
Fluid Mechanics
Thermodynamics
General Aptitude